Relation between Left Atrial Remodeling in Young Patients with Cryptogenic Stroke and Normal Inter-atrial Anatomy
Article information
Abstract
Background and Purpose
To investigate an association between left atrial (LA) structural and P wave dispersion (PWD) during sinus rhythm, and electrical remodeling in cryptogenic stroke (CS) patients.
Methods
Forty CS patients and 40 age- and sex-matched healthy controls were enrolled. P wave calculations were based on 12-lead electrocardiography (ECG) at a 50-mm/s-paper speed with an amplitude of 10 mm/mV. Difference between the maximum and minimum P wave duration was the P wave dispersion (PWD=Pmax-Pmin). LA deformation was evaluated by speckle tracking echocardiography within 3 days of the acute event.
Results
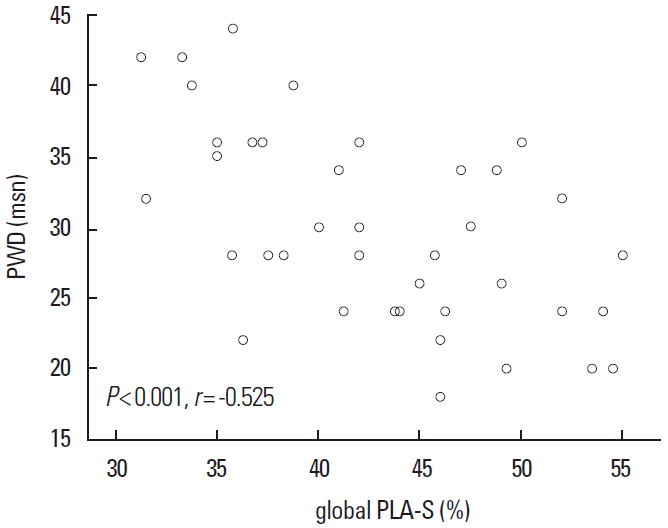
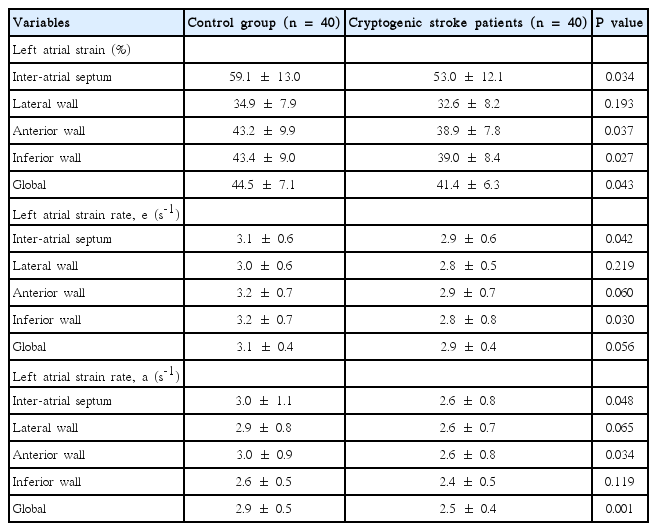
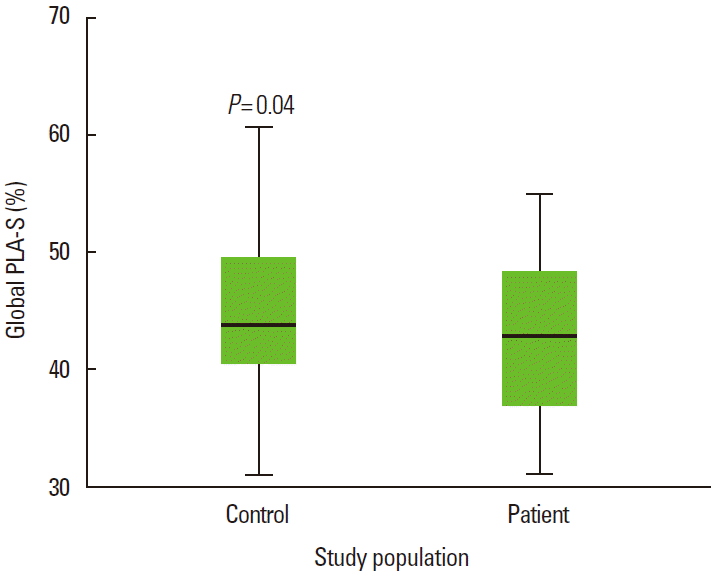
PWD was 30.1±7.0 ms and 27.4±3.5 ms in CS and control group (P=0.02), whereas LA maximum volume index [LAVImax] was 20.4±4.5 mL/m2 and 19.9±2.4 mL/m2 in CS and control group, respectively (P = 0.04). While global peak LA strain was [pLA-S] (LA reservoir function) 41.4 ± 6.3% and 44.5 ± 7.1% in CS and control group, (P = 0.04), global peak late diastolic strain rate values [pLA-SRa] (LA pump function) were 2.5 ± 0.4% and 2.9 ± 0.5% in CS and control group, respectively (P = 0.001). A mild and a strong negative correlation between global pLA-S and LAVImax (r=-0.49; P<0.01), and between PWD and global pLA-S (r = -0.52; P < 0.01), respectively, was observed in CS.
Conclusions
Increased PWD is associated with impaired LA mechanical functions and enlargement, and involved in the pathophysiology of AF or an AF-like physiology in CS.
Introduction
Cryptogenic stroke (CS) refers to a cerebral ischemia of unknown origin [1]. While atrial fibrillation (AF) is the most common associated cardiac arrhythmia making up to 12% of strokes among the general population [2], asymptomatic paroxysmal AF (PAF) is often suspected as the underlying cause of CS [3]. The progressive nature of AF is well established and known to lead to electro-anatomical remodeling and atrial fibrosis [4]. Atrial fibrosis develops from complex interactions among several cellular and neurohumoral mediators, with the resultant fibrotic tissue causing a low a voltage response and other electrophysiological changes in the left atrium (LA) that act as a substrate for the occurrence of AF. While electrical remodeling may shorten atrial refractoriness and contributes to an increase in the stability of AF, atrial structural remodeling occurs because of heart failure and other underlying cardiovascular diseases. Moreover, LA fibrosis has been associated with an increased risk of developing a stroke in the absence of AF or PAF, as shown by magnetic resonance imaging (MRI) [5].
Speckle tracking echocardiography, a new imaging technique that offers offline calculation of myocardial velocities and deformation parameters such as strain and strain rate (SR), provides important insights into systolic and diastolic function, ischemia, myocardial mechanics, and many other pathophysiological heart processes [6-9]. A sensitive, noninvasive, reproducible, and quantitative assessment of LA longitudinal deformation, which is closely related to LA physiology and anatomy, is achieved by means of this technique [6-9].
LA longitudinal deformation indices (global peak LA strain [pLA-S], global peak LA early diastolic strain rate [pLA-SRe], and global peak LA late diastolic strain rate [pLA-SRa]) appear to provide the most accurate physiologic and anatomic analysis of LA.
As decreased LA longitudinal deformation has been closely associated with PAF, its detection allows the identification of patients with a high risk of AF recurrence after catheter ablation procedures, irrespective of LA enlargement [9,10]. Furthermore, decreased LA longitudinal deformation predicts the extent of LA fibrosis independent of other echocardiographic parameters, including rhythm [11,12]. Therefore, characterization of LA fibrosis using speckle-tracking echocardiography may aid in the diagnosis of CS.
Recently, an association between a decreased LA ejection fraction (LA EF) and an increased risk of PAF in CS has been suggested [13]. Furthermore, LA active relaxation and contraction is lower in PAF patients than in those with sinus rhythm, regardless of LA enlargement and aging.
P wave dispersion (PWD), defined as the difference between the maximum (Pmax) and the minimum (Pmin) P wave duration on electrocardiography (ECG), has been regarded as an independent risk factor for the development of AF [14,15]. As PWD may be easily measured using a single ECG, it is considered an electrocardiographic marker of atrial electromechanical dyssynchrony caused by the heterogeneous propagation of sinus impulses. On the other hand, PWD determined within the first day after of an acute ischemic stroke serves as a surrogate marker to predict PAF and the risk of recurrent strokes [16].
The aim of this study was to investigate associations between LA electro-structural remodeling and the occurrence of CS.
Methods
Patients and data collection
In this cross-sectional study, we have used speckle tracking echocardiography and ECG to assess LA structural and electrical remodeling, and examined its association with the occurrence of CS.
All consecutive patients presenting with a stroke of unknown origin despite extensive routine diagnostic testing and diagnosed with CS between 2009 and 2010, were included in the study. Strokes were classified according to the TOAST criteria [17].
We excluded patients who had a previous cerebrovascular event, a TOAST Classification of High- and Medium-Risk Sources of Cardioembolism, or malignant arterial hypertension; uncontrolled diabetes mellitus; a history of effort angina, acute coronary syndrome, or revascularization procedures; evidence of a positive exercise stress test before the acute stroke; segmental wall abnormalities at echocardiography; presence or 24-hourholter detected AF, atrial flutter, or other major arrhythmias; moderate-to-severe valvulopathies, existence of inter-atrial block on 12-lead ECG, or inter-atrial septum abnormalities; patients who had a malign clinical course in the neurology intensive care unit, including severe kidney, respiratory, cardiac or hepatic failure, and inadequate acoustic windows were excluded. Patients who required intubation because of respiratory insufficiency, and those who required more than 3 days in a neurology intensive care unit were also excluded.
All patients fulfilled both the inclusion and exclusion criteria. From those, 40 (aged between 18 and 55 years) who were admitted to our emergency department and hospitalized in the neurology intensive care unit with a diagnosis of acute ischemic stroke, and a control group comprised of 40 age- and sex-matched healthy individuals, were enrolled in the study. Participants in the control group underwent a complete routine clinical and cardiac laboratory evaluation for the detection of occult cardiac disease, including 12-lead ECG, transthoracic/transesophageal echocardiography (TTE/TEE), extra cranial arteries duplex sonography, and single ECG-24-hour Holter ECG monitoring before inclusion in the study. Individuals with any cardiac structural pathology and arrhythmia, including patent foramen ovale, atrial septal aneurysm, or paroxysmal atrial fibrillation, were excluded from the study.
The following routine diagnostic tests were performed in all patients: cranial computed tomography and magnetic resonance imaging of the brain, or both. Duplex sonography of the extra cranial and intracranial arteries, a single ECG-24-hour Holter ECG monitoring, and TTE/TEE were performed in patients within a median of 3 days after the acute stroke.
LA structural remodeling analysis using conventional and speckle tracking echocardiography
Electrocardiogram-guided echocardiographic measurements were carried out using a commercially available ultrasound device (ie33, Philips Medical System, Bothell, Washington, USA). Images were obtained from parasternal and apical windows using 2D, M-Mode, and Doppler echocardiography.
The LVEF, as a standard index of global LV systolic function, was measured using the Simpson’s method. The ratio between the peaks of early (E) and late (A) diastolic LV filling velocities was used as the standard index of LV diastolic function [18]. Tissue Doppler measurements were obtained at end-expiration with the sample volume placed on the atrial side of the mitral annulus at the basal inter-atrial septum in the apical four-chamber view. Both early diastolic (E′), and late diastolic (A′) annular velocities were obtained. The E/E′ ratio was also calculated to provide a reliable index of the LV filling pressure.
At end-systole, just before the opening of the mitral valve (at the end of the T wave on the ECG), the LA maximum volume was indexed to body surface area (LAVImax). At end-diastole, just before mitral valve closure (at the beginning of the QRS complex on the ECG), minimum LA volume to body surface area (LAVImin) were measured and indexed to body surface area, as previously described [19].
For speckle tracking echocardiography analysis, apical 4- and 2-chamber view images were obtained using conventional 2-dimensional gray-scale echocardiography during breath-hold. Care was taken to obtain a reliable delineation of the atrial endocardial border. The frame rate was set at > 100 frames/s. Three consecutive heart cycles were recorded in digital format for offline analysis using the commercially available software QLAB 6.0 (Philips Medical System, Bothell, Washington, USA). Segments for which adequate tracking quality could not be obtained despite manual adjustment, were excluded from analysis, and patients in whom some segments were excluded due to the impossibility of adequate tracking, LA deformation parameters were calculated by averaging the values measured in the remaining segments. LA peak longitudinal strain and strain rate at basal segment from the apical views of the inter-atrial septum, LA lateral wall, LA anterior wall, and LA inferior wall in the 5 × 3 mm region of interest were calculated as described in current guidelines [8]. LA reservoir strain during systole was obtained at the time of aortic valve closure, and LA contractile strain rate during late diastole was obtained at the onset of the P wave on ECG. LA conduit strain rate during early diastole was obtained as previously described. The reproducibility and feasibility of speckle tracking echocardiography measurement of LA longitudinal strain and strain rates were considered acceptable.
LA electrical remodeling analysis using P wave dispersion
All standard 12-lead ECGs were obtained simultaneously at a 50-mm/s-paper speed and amplitude of 10 mm/mV. Two cardiologists blind to the clinical status of the patients performed the P wave analysis with calipers and magnifying glasses to decrease the risk of measurement errors. The onset of the P wave was defined as the point in which the wave showed the first visible upward and downward departure from baseline for positive and negative waveforms, respectively. A return to the baseline was considered the end of the P wave. Maximum P wave duration (Pmax), measured from any of the 12 leads of the surface ECG, was used for the longest atrial conduction time. The difference between the maximum and minimum P wave duration was defined as PWD (PWD = Pmax-Pmin). Intraobserver and interobserver coefficients of variation were acceptable. Written informed consent was obtained from each patient. The local ethics committee approved the study.
Statistical analysis
Data have been presented as mean ± SD for continuous variables and as proportions for categorical variables. For continuous data, statistical differences were evaluated using a Student’s t test, or alternatively a Mann-Whitney U test for cases in which the assumptions of the Student’s t test were not satisfied. For categorical data, the chi-square test was used. All correlations were analyzed using a Spearman’s rank correlation test. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software, version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Clinical and conventional echocardiographic characteristics of the study population are shown in Table 1. Patients with high blood pressure and high glucose levels received appropriate medication. No significant differences with regard to age, gender, body mass index, body surface area, heart rate, blood pressure, left atrial minimal volume index, and left ventricular ejection fraction, were observed between the two groups. The PWD was 30.1 ± 7.0 ms and 27.4 ± 3.5 ms in the CS and control group, respectively (P= 0.02), whereas the LA maximum volume index [LAVImax] was 20.4 ± 4.5 mL/m2 and 19.9 ± 2.4 mL/m2 in the CS and control group, respectively (P= 0.04). The global peak LA strain was [pLA-S] (LA reservoir function) 41.4 ± 6.3% and 44.5 ± 7.1% in the CS and control group, respectively (P= 0.04), whereas the global peak late diastolic strain rate values [pLASRa] (LA pump function) were 2.5 ± 0.4% and 2.9 ± 0.5% in the CS and control group, respectively (P= 0.001). A strong negative correlation between global pLA-S and LAVImax (r= -0.49; P< 0.01), as well as a moderate correlation between PWD and global pLA-S (r= -0.52; P< 0.01), was found in CS patients (Figures 1, 2). Although there was no significant difference in left atrial tissue Doppler velocities between patients and healthy controls, global pLA-S was found to be significantly lower in CS patients (Table 2). A significant difference was found in the pattern of LA segment changes in CS patients (Table 2 and Figure 3). The LA reservoir function (LA strain) was smaller in CS patients than in healthy controls. The inter-atrial septal and inferior wall early diastolic strain rates (LA conduit function) and the anterior late diastolic strain rate (LA pump function) were significantly lower in CS patients than in healthy controls, whereas the interatrial septal late diastolic strain rate tended to be lower but the difference was not statistically significant.

A strong negative correlation between global pLA-S and LAVImax (r=-0.49; P<0.01) was found in CS patients.
Discussion
In this study we have demonstrated that electrical remodeling, shown by an increase in PWD on surface ECG, was associated with structural remodeling and decreased LA deformation shown on echocardiography. These findings suggest that LA structural, functional, and electrical impairments in conjunction with other risk factors i.e. increased thrombogenicity and a heightened proinflammatory response, may ultimately result in cardioembolism in patients without inter-atrial septal abnormalities. PWD was increased in CS patients mainly due to an increase in maximum P wave values rather than a decrease in minimum P wave durations on a 12-lead ECG, and it was associated with an increased risk of AF development. Although, the increase in PWD and structural changes observed in LA may explain the occurrence of CS, whether these changes are an underlying cause or an associated finding in the early post-stroke period (within 1 week) in CS patients, remains unclear [18-20].
Furthermore, AF results in LA remodeling through a process that involves the deposition of fibrotic tissue, leading to changes in the electrophysiological properties of the LA substrate. Detection and quantification of LA structural remodeling as a possible determinant of fibrosis, is now possible using delayed-enhancement magnetic resonance imaging. Nevertheless, whether substrate changes seen in AF patients are related to stroke and current stroke risk stratification schemes is not yet clear.
The prolongation of intra- and inter-atrial conduction times and the inhomogeneous propagation of sinus impulses are well known electrophysiological characteristics seen in patients with PAF [15,16]. PWD has proven to be a sensitive and specific ECG predictor of AF in various clinical settings, including patients with hypertension, coronary artery disease, undergoing coronary artery bypass surgery, or obstructive sleep apnea [20-23]. Increases in PWD and Pmax are important indicators of subsequent PAF attacks and a tendency towards persistent atrial fibrillation. Furthermore, an association between incremental LA enlargement and an increased risk of AF during follow-up has been suggested, with the risk of developing AF being four times greater in patients with enlarged LA [4,5]. Results from studies by Abhayaratna et al. [24] showed that increased LAVImax was an independent predictor of death, heart failure, atrial fibrillation, and ischemic stroke.
The role of LA function in stroke pathogenesis has been supported by recent data showing that inter-atrial septal abnormalities lead to LA dysfunction, which may in turn contribute to LA thrombosis and subsequent ischemic stroke [25,26]. Moreover, Goch et al. [27] showed that patients with only atrial septum aneurysm (ASA) had depressed LA systolic function and increased left atrial appendage function, which might be a compensatory mechanism for LA deterioration. Jin et al. revealed that LA active pump function was significantly depressed, and closely correlated with left atrial appendage emptying in CS patients with ASA alone [3]. Therefore, impaired LA and LA appendage (LAA) functions may be crucial pathophysiologic mechanisms for ischemic stroke in patients with ASA alone.
To our knowledge, LA structure and function in CS patients without inter-atrial septal abnormalities have not been thoroughly investigated. The present study provides new insights into LA remodeling in patients with CS, demonstrating that global pLA-S, a newer parameter of atrial function, is more sensitive than the traditional echocardiographic markers of LA size and function used to detect LA remodeling.
Routine measurement of LA strain might guarantee the detection of LA remodeling, and provide useful additional information for CS diagnosis. Thus, this novel imaging method may be considered a promising index for a more accurate quantification of LA function, allowing the potential identification of LA impairment, which may be a useful parameter for CS risk stratification. However, as this novel technique has insufficient resolution to measure the radial strain of the thin-walled LA, LA deformation assessment is only based on longitudinal strain. Longitudinal strain is positive during the reservoir period, in which LA myocardial fibers relax and stretch to adapt to the incoming blood flow, negative during the pump and most of the conduit phase, during which the LA is emptying, and flat during diastasis, corresponding to the late phase of the conduit phase. Therefore, measurements taken at end-systole, as well as early and late end-diastole, may provide information about the reservoir, conduit, and pump functions. Furthermore, global pLA-S has considerably high diagnostic accuracy while the E/E′ ratio correlates poorly with invasively measured LA pressure. In patients with AF who underwent catheter ablation, the systolic, as well as the early and late diastolic strains, were considerably lower in patients with persistent AF than in patients with PAF or normal subjects [10]. Similarly, lower strain and strain rate were associated with a higher rate of AF reoccurrence after catheter ablation, and an improvement in LA strain correlated with a reversion of LA remodeling in patients who underwent catheter ablation for AF. This latter finding may have essential prognostic and therapeutic implications concerning the post procedure cardioembolic risk and oral anticoagulation therapy.
Despite the obvious advantages of two-dimensional echocardiography, the difficulty of endocardial border tracing and its reliance on geometrical assumptions, which ignore LV and LA geometrical differences between individuals, limits its application. However, LA strain measurements may facilitate atrial remodeling assessment in various pathological conditions, as well as reverse remodeling after medical or more invasive therapy, such as cardiac resynchronization therapy, ablation for atrial fibrillation or flutter, hypertrophic cardiomyopathy, or coronary artery diseases [28-31]. In support of this view, Karabay et al. [32] recently demonstrated that LA deformation parameters measured by speckle tracking were found to predict impaired LA appendage (LAA) functions and the presence of LAA thrombus in ischemic stroke patients with suspected cardioembolism in sinus rhythm. In that study, LA reservoir and pump functions were found to be significantly lower in patients with LAA thrombus, and the LA reservoir function showed the strongest correlation with LAA morphologic parameters.
Pagola et al. [33] recently claimed that measurement of pLA-S in patients with CS might be a useful tool to detect patients with occult PAF. Furthermore, they concluded that PLA-S analysis may play a role in the selection of patients with normal size LA for prolonged cardiac monitorization, because most patients with PAF showed low LAS in their study. Although, their results were similar, the patients mean age of the study population was 62 years, whereas in our study it was only 42 years. Since aging is associated with atrial fibrosis and atrial arrhythmias [34], we have excluded an aging effect on left atrial anatomy and function in CS patients in this study. For the first time, we have offered LA remodeling in young CS patients.
LA structural remodeling, as demonstrated by the histopathology findings, significantly influences global pLA-S [35]. Therefore, assessment of LA function and LA ultrastructural changes may provide additional information for the prediction of cardiovascular events. In particular, atrial fibrosis has been strongly associated with the presence of heart disease and arrhythmias, including congestive heart failure and atrial fibrillation. Moreover, an adverse prognosis linked to progressive LA substrate remodeling, showed a correlation between stroke and high levels of LA fibrosis, detected by delayed enhancement MRI in patients with AF. However, global pLA-S measured by speckle tracking echocardiography was negatively correlated with LA myocardial fibrosis grade, while poorer correlations with the LA indexed volume, LA ejection fraction, and E/E′ ratio have been shown.
As global pLA-S is also correlated with endocardial thickening, a histologic alteration appearing in the earlier stages of structural remodeling [35], it can be used to assess an increase in interstitial fibrosis in conditions which compromise the elastic properties of the atrial myocardium, inevitably leading to impairment of atrial compliance and reduction of LA reservoir function.
Although our findings are in general agreement with the results of previous studies, there are several notable differences. Compared with our study, most studies selected subjects from the general population who presented inter-atrial septal abnormalities. Therefore, their results are not representative of all the underlying pathophysiologic mechanisms operating in the development of ischemic events in CS patients with no inter-atrial septal abnormalities. Furthermore, in order to eliminate possible etiologies associated with paradoxical embolism and to prevent misleading results, we only included CS patients without PFO or ASA on TEE.
Limitations
The main limitation of our study the inability to demonstrate a clear relationship between LA structure and LA strain using histopathological or delayed enhancement MRI.
Secondly, to exclude undetected paroxysmal AF (PAF), in addition to a single 24-hour Holter monitoring in all subjects, we performed standard 12-lead ECGs at every visit as well as at any time if the subjects reported palpitations. However, we were not able to exclude PAF episodes reliably because they are often asymptomatic, and those undetected episodes may have contributed to confounding during the statistical analysis of LA function.
Although the effect of various drugs, including anti-thrombotic drugs, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and statins, on LA function or plasma biomarkers could not be fully controlled, as not all subjects were receiving the same drug therapies, there were no significant differences in medication regimens between the two study groups.
Some cardiac abnormalities that cannot be detected by routine diagnostic methods may be a cause of AF or PAF and subsequent CS [36].
Acute cerebral events such as subarachnoid hemorrhage may cause stunned myocardium, a neurogenic cardiac dysfunction, as well as arrhythmia and ischemia changes during the acute phase. Although the mechanisms responsible for those changes are unknown, the release of stress hormones, adrenaline, and nor-adrenaline, caused by major stressful events, is suspected [37,38].
Lastly, we cannot completely exclude the existence of right-to-left shunt, as exclusion of PFO was only confirmed by TEE using contrast bubbles. Recently, transcranial Doppler examination has been considered more sensitive than TEE for the diagnosis of a right-to-left shunt. Nevertheless, our hypothesis that LA dysfunction itself may contribute to thrombosis in situ through an embolism with an extra-cardiac origin remains plausible. Therefore, further interventional studies are warranted to investigate whether improvement of LA function by reversion of LA remodeling would protect from ischemic stroke in the general population.
Conclusions
Increased PWD on surface ECG is associated with left atrial mechanical remodeling and dysfunction, both of which may be substrates for thromboembolism in CS patients.
Notes
The authors have no financial conflicts of interest.