Mechanical Thrombectomy for Acute Ischemic Stroke in Metastatic Cancer Patients: A Nationwide Cross-Sectional Analysis
Article information
Abstract
Background and Purpose
Mechanical thrombectomy (MT) is the standard treatment for large vessel occlusion (LVO) acute ischemic stroke. Patients with active malignancy have an increased risk of stroke but were excluded from MT trials.
Methods
We searched the National Readmission Database for LVO patients treated with MT between 2016–2018 and compared the characteristics and outcomes of cancer-free patients to those with metastatic cancer (MC). Primary outcomes were all-cause in-hospital mortality and favorable outcome, defined as a routine discharge to home (regardless of whether home services were provided or not). Multivariate regression was used to adjust for confounders.
Results
Of 40,537 LVO patients treated with MT, 933 (2.3%) had MC diagnosis. Compared to cancer-free patients, MC patients were similar in age and stroke severity but had greater overall disease severity. Hospital complications that occurred more frequently in MC included pneumonia, sepsis, acute coronary syndrome, deep vein thrombosis, and pulmonary embolism (P<0.001). Patients with MC had similar rates of intracerebral hemorrhage (20% vs. 21%) but were less likely to receive tissue plasminogen activator (13% vs. 23%, P<0.001). In unadjusted analysis, MC patients as compared to cancer-free patients had a higher in-hospital mortality rate and were less likely to be discharged to home (36% vs. 42%, P=0.014). On multivariate regression adjusting for confounders, mortality was the only outcome that was significantly higher in the MC group than in the cancerfree group (P<0.001).
Conclusion
LVO patients with MC have higher mortality and more infectious and thrombotic complications than cancer-free patients. MT nonetheless can result in survival with good outcome in slightly over one-third of patients.
Introduction
Acute ischemic stroke (AIS) is common in patients with active malignancy, occurring in 15% of all cancer patients [1]. The incidence of cancer-related AIS is proportional to survival time, which exposes patients to an increased risk of cancer-related hypercoagulability [1].
Patients with cancer tend to be excluded from receiving tissue plasminogen activator (tPA) as they carry a high risk for bleeding secondary to brain metastases, thrombocytopenia, coagulopathy, therapeutic anticoagulation, and recent surgeries [2,3]. Given these limitations, understanding the outcomes and risks of mechanical thrombectomy (MT) in cancer patients may help make decision for MT.
Patients with cancer and reduced life expectancy were excluded or underrepresented from the seminal clinical trials that established the efficacy of MT for large vessel occlusion (LVO) stroke [4-8]. Several small single-center cohort studies have addressed MT in cancer patients, with the number of patients treated with MT ranging from 19 to 27 [9-12]. These studies have demonstrated that MT is feasible, but have shown inconsistent results with regard to procedural effectiveness, mortality, disability, and the rate of hemorrhagic transformation compared to non-cancer MT-treated patients [9-12]. In a more recent analysis of the multicenter European MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in The Netherlands) registry; 4.8% of patients undergoing MT had active cancer [3]. Despite similar technical success, cancer patients had higher mortality and greater disability than cancer-free patients, but nonetheless about one-quarter of the patients regained functional independence.
Our study aimed to investigate the outcomes and in-hospital complications of patients with metastatic cancer (MC) treated with MT for LVO AIS in a large, cross-sectional USA nationwide database. Our hypothesis was that thrombectomy in MC patients results in a comparable rate of discharge to home compared to cancer-free LVO patients, despite higher in-hospital mortality.
Methods
Data source
This is a retrospective cohort study using the Agency for Healthcare Cost and Utilization Project (HCUP) Nationwide Readmission Database (NRD) for the years 2016–2018 [13]. The NRD is the largest publicly-available all-payer inpatient health care readmission database in the USA. The NRD is drawn from HCUP state inpatient databases containing verified patient linkage numbers that can be used to track a person across hospitals within a state while adhering to strict privacy guidelines. It provides longitudinal information about patients’ initial hospitalization and subsequent readmissions in a calendar year. It contains a weighted sample of hospitalizations in the USA, which can be used to derive national estimates of various hospitalizations directly. All authors who worked directly with the data set underwent a data handling and training module required by HCUP. Data are available to be shared by the authors upon reasonable request. A checklist of the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) items to be reported in observational studies using routinely collected health data is provided in Supplementary Table 1. Due to the anonymous nature of this administrative database of hospital admissions, neither local institutional review board nor patient consent were required.
Study population
Our primary study population was all USA admissions for incident AIS who underwent MT during the 3-year study period. Index admission was defined as the first admission with the primary diagnosis of AIS during the study period; subsequent admissions for stroke were not included. Admissions were excluded if age was <18 years, the patient had a prior history of stroke, or the file had missing data. In the NRD, patient identifiers cannot be linked across years; hence index admissions on October 1st or beyond were excluded to allow a minimum of 3 months of follow-up. In accordance with the HCUP data user agreement, we excluded reporting any variables containing a small number of observations (≤10) that could pose a risk of personal identification or a data privacy violation.
For analysis, we divided the cohort into two groups. We selected MC as the diagnosis of interest to ensure that we would focus on a more homogeneous population of cancer patients with advanced disease. Patients with a cancer diagnosis without metastases were excluded to allow us to focus only on patients with the most severe disease. The diagnosis of interest (n=933) was patients with MC and LVO stroke who were treated with MT. This group was compared to LVO stroke cancer-free patients who were treated with MT (n=38,166). International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) codes were utilized to capture the diagnoses of interest, including MC (Supplementary Table 2). An independent audit comparing ICD-10 codes to hospital discharge records showed that the former was highly reliable and accurate [14].
Baseline illness and stroke severity
Baseline demographic data including age and sex were collected. Race was not included as it is not available in the NRD. Baseline illness severity was determined using All Patient Refined Diagnosis Related Group (APR-DRG) subclasses. This system uses weighted diagnostic codes to capture admission diagnosis, complications, and risk of mortality at discharge, and classifies patients into four clinically meaningful severity groups ranging from 1 (mild illness) to 4 (extreme illness) [15]. Stroke severity was determined as the presence of one or more of the following elements: paralysis/paresis, aphasia, coma, cerebral edema, cerebral herniation, and/or requiring mechanical ventilation, in accordance with prior published research [16].
Study outcomes
The primary outcomes were all cause in-hospital mortality and favorable outcome, which was defined as a routine discharge to home (regardless of whether home services were provided or not). Secondary outcomes included hospital length of stay, non-home discharge (defined as non-routine discharge to a rehabilitation hospital; a short, intermediate, or long term nursing facility; or hospice), development of intracerebral hemorrhage (ICH), the need to undergo decompressive hemicraniectomy, and 90-day hospital readmission. Tertiary outcomes included the frequency of a variety of acute hospital complications, which were captured by their corresponding ICD-10 codes (Supplementary Table 2). In-hospital mortality and length of stay are directly coded in the NRD.
Statistical analysis
We compared MT-treated patients with MC and MT-treated cancer-free patients. Continuous variables were compared using Student’s t-test, and categorical variables were compared using the χ2 test. Multivariate linear and logistic regression analyses were used to adjust for confounders (age, sex, severe stroke, APR-DRG=4, and tPA use) and calculate adjusted odds ratio (aOR) for the association of MC with all primary and secondary outcome measures. To explore the independent role of MC in explaining death or discharge to a facility after MT, we constructed a stepwise backward regression model accounting for demographics, stroke and disease severity, and specific complications and procedures. Admissions that ended with in-hospital mortality were excluded from readmissions analysis. All P-values were 2-sided, with 0.01 as the threshold for statistical significance to adjust for multiple comparisons. Data Analysis was performed using Stata version 17 (StataCorp., College Station, TX, USA).
Results
Our analysis identified 1,056,011 eligible patients admitted for AIS during the 3-year study period, 40,537 (3.8%) of whom underwent MT (Figure 1). Of those who underwent MT, 933 (2.3%) had MC. The different types of cancer that were represented are shown in Supplementary Table 3.

Cascade diagram showing patient grouping. Of all acute ischemic stroke (AIS) patients who underwent thrombectomy, the primary analysis compared 933 patients with 38,166 patients with no cancer. The 1,438 patients with a cancer diagnosis but no metastases were excluded. The secondary analysis of metastatic cancer patients compared the 933 thrombectomy-treated patients with 25,731 patients treated medically for AIS.
We compared the 933 MT-treated patients with MC to 38,166 cancer-free stroke patients treated with MT (Table 1). Stroke severity was highly comparable between the two groups. MC patients were less likely to receive tPA (13% vs. 23%, P<0.001) but had a similar frequency of ICH (about 20%). MC patients had a higher frequency of severe medical illness based on APR-DRG coding (52% vs. 39%, P<0.001). Specific medical complications that occurred more frequently in the MC cohort included pneumonia, sepsis, deep vein thrombosis, pulmonary embolism, and acute coronary syndromes (all P<0.001). MC patients were significantly less likely to undergo placement of a percutaneous endoscopic gastrostomy (PEG) tube or tracheostomy. The frequency of hemicraniectomy was <1% in both groups.
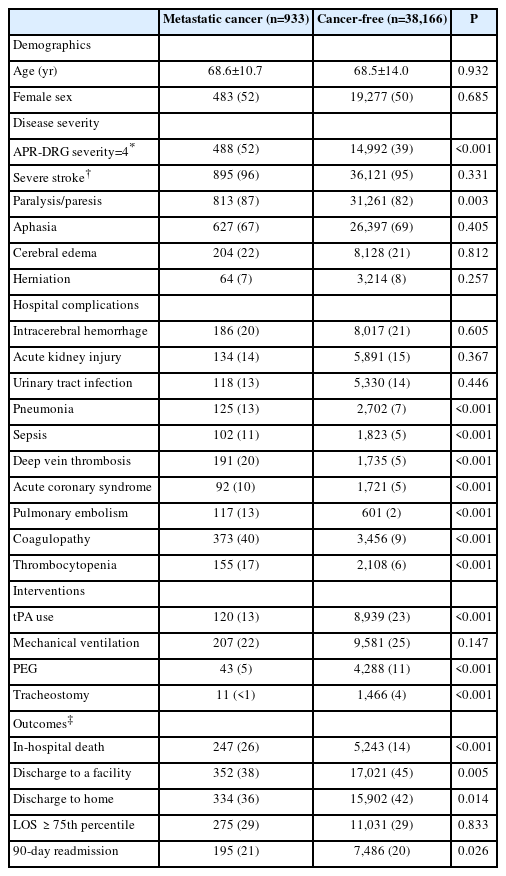
Hospital complications, interventions, and outcomes among stroke patients treated with mechanical thrombectomy, with or without metastatic cancer
In-hospital mortality was significantly higher in MC patients (26% vs. 14%, P<0.001). The overall proportion of MC patients with a favorable outcome (discharged to home) was slightly lower, but highly comparable to the cancer-free group (36% vs. 42%, P=0.014). Hospital length of stay and 90-day readmission rates were similar. On multivariate regression adjusting for confounders, the only outcome variable that was significantly different between the MC and cancer-free groups was mortality (Table 2). Among cancer patients who underwent thrombectomy, there was no significant difference in mortality between those with or without brain metastases.

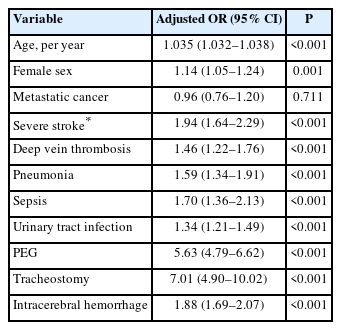
Multivariate logistic regression analysis comparing mechanical thrombectomy-treated stroke patients with versus without metastatic cancer
To explore the independent role of MC in explaining death or discharge to a facility among MT-treated stroke patients, we created a stepwise backward multivariate logistic regression model that included demographics, MC, stroke and illness severity, and specific complications and procedures (Table 3). After accounting for ten variables that were significantly associated in the final model, the adjusted odds ratio for the association between MC and poor outcome was nonsignificant (Table 3). Variables that did predict death or discharge to a facility included age, female sex, overall medical disease severity, severe stroke, deep vein thrombosis, pneumonia, sepsis, ICH, urinary tract infection, and placement of a PEG or tracheostomy. There was no interaction between MC and overall disease severity (APR-DRG=4) with regard to mortality.
Discussion
AIS is the second most common complication that involves the central nervous system in cancer patients, the first being cerebral metastases [17]. Viewed another way, one-in-ten patients with AIS have a history of remote or active cancer, making it critical to establish the best management strategies for LVO in this vulnerable group of patients [18]. In an autopsy study, it was reported that almost 15% of cancer patients had a stroke on post-mortem examination, with hemorrhagic and ischemic strokes being equally prevalent [1].
Hypercoagulability in patients with cancer is hypothesized to be due to tumor cell-derived cytokines, elevated D-dimer levels, tumor embolism, chemotherapy, as well as vascular risk factors like diabetes and hypertension [9,10,19], and it is believed that embolism secondary to hypercoagulability is the most important cause of stroke in cancer patients [20]. In a recent retrospective cross-sectional study, the most common stroke mechanism in cancer patients was cryptogenic (embolic stroke of unknown source), followed by large vessel disease [17,21]. Conventional stroke risk factors such as hypertension, diabetes, and atrial fibrillation are underrepresented in cancer-related stroke, invoking hypercoagulability and other cancer-specific causes of stroke [10,22,23].
Stroke patients with cancer are often excluded from receiving thrombolytic therapy due to concerns about brain metastasis, thrombocytopenia, coagulopathy, recent surgery, or overall poor prognosis [24,25]. That is reflected in our study, as significantly fewer MC patients received bridging lytic therapy than cancer-free patients (13% vs. 23%, P<0.001), despite similar stroke severity. The rate of ICH was similar (about 20%) in MC and non-MC patients treated with thrombectomy.
The most important question addressed by our study is whether it is worthwhile and beneficial to perform MT in MC patients who experience LVO stroke, given their poor prognosis and comorbidities. The two groups were highly comparable with regard to age, sex, and stroke severity (Table 1). By contrast, the MC patients had significantly higher comorbidity indices overall, including higher rates of infection (pneumonia, sepsis) and thrombosis (acute coronary syndrome, DVT, and pulmonary embolism). Hospital length of stay and all-cause 90-day readmission rates were similar.
In-hospital death was significantly greater in the MC cohort treated with thrombectomy (26% vs. 14%). It is also notable that tracheostomy and PEG were performed significantly less frequently in MC patients, presumably due to more withholding of life sustain therapy. Despite this, the frequency of home discharge was only slightly lower in MC versus non-MC patients: 36% versus 42% overall, which translates into a 49% versus 48% rate of discharge home among those who survived their hospitalization. These encouraging results are consistent with other studies that have addressed clinical and safety outcomes in patients with AIS and active cancer after endovascular treatment. Of 2,583 stroke patients who underwent MT in the MR CLEAN registry, 4.8% had active cancer (diagnosed <12 months prior to stroke, with metastases, or under active treatment), which is comparable to the 2.3% frequency of MC that we observed [3]. In this study, successful reperfusion and ICH rates did not differ between cancer and cancer-free patients. Other studies have also shown that there is no difference in recanalization rates, procedural time metrics, or choice of anesthesia in cancer compared to cancer-free patients who undergo thrombectomy [26-28]. Although mortality was higher and functional outcome was worse in the cancer cohort at 90 days, about 25% had regained functional independence [3].
A smaller single-center study from Korea reported a 7.1% frequency of current malignancy out of a total of 378 AIS patients treated with MT [11]. There was no difference in the rate of good functional outcome (37% vs. 40%) or ICH between those with and without cancer. Other smaller single-center registries and case-control studies have reported a frequency of cancer among MT-treated stroke ranging from 2.7% to 10.3%, and inconsistent results regarding procedural success, the risk of ICH, and clinical outcome compared to cancer-free patients [9-12,29-31]. In our secondary analysis, we showed that 3.5% of MC patients hospitalized for AIS were treated with MT, which is comparable to the rate of 3.8% among cancer-free stroke patients (Figure 1).
Our study has many limitations. First, inherent to the use of administrative databases such as the NRD, diagnostic accuracy may be affected by coding errors, the absence of detailed neurological and imaging data, and the lack of independent auditing and verification of key outcome measures. Also, temporal relationships that occur during hospitalization cannot be elucidated. Second, the NRD lacks data on stroke and medical illness severity scales, location and size of ischemic lesions, baseline or discharge destination, procedural success, timing of acute treatment, concurrent medical treatments such as anticoagulation, and long-term functional outcome. Hence, the results of our regression analysis should be interpreted with caution. Third, we could not distinguish between mortality as a result of stroke complications or from mortality due to withdrawal of care due to patients and family wishes. Fourth, our results are applicable to patients with advanced cancer with metastases, and may not accurately reflect the clinical course of stroke patients with early stage non-MC. Fifth, MC patients were less likely to undergo PEG or tracheostomy placement, which may have confounded the outcome, most likely reflecting decisions to pursue comfort care. Sixth, the NRD does not link patients identifiers across years. Seventh, in the NRD it is not possible to differentiate whether a diagnosis of MC is a new or historical diagnosis. Finally, discharge to home hospice is included under the routine discharge to home classification (inpatient hospice is in a separate category under non-routine discharge) in the NRD, which does not constitute a favorable outcome as we defined it. However, discharge to home hospice is rare in our clinical experience. Despite these limitations and lack of granularity in the NRD, administrative database studies offer strength in numbers, generalizability, and insight into real-world practices, especially for uncommon conditions in which randomized clinical trials are not practical to conduct.
Conclusion
In summary, MC patients with LVO stroke treated with MT are discharged home at a similar rate to cancer-free patients, suggesting that they can benefit from the procedure. Given the many limitations of an administrative database such as the NRD, this analysis should be viewed as preliminary and inconclusive. Pending the publication of more detailed prospective registries and clinical trials, our data may hopefully help clinicians, patients, and caregivers alike make more thoughtful and informed decisions regarding the risks and benefits of MT in stroke patients with MC.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.02334.
The RECORD statement: checklist of items, extended from the STROBE statement, that should be reported in observational studies using routinely collected health data*
International Classification of Disease, Tenth Revision (ICD-10) codes
Breakdown of cancer types
Notes
Disclosure
S.A.M. receives consulting fees from Biogen for effort as a member of the independent safety monitoring committee for the CHARM trial. The other authors have no relationships to disclose.
Acknowledgements
Dr. Aboul-Nour created the idea of the project, contributed to methodology, design, statistical planning, and manuscript writing. Dr. Maraey contributed by analyzing the NRD data, statistical planning and analysis. Dr. Jumah contributed to methodology and design, literature review, and manuscript writing. Dr. Khalil contributed by statistical planning and analysis, ICD-10 codes extractions, and revision. Dr. Elzanaty contributed by analyzing the NRD data. Dr. Elsharnoby contributed by analyzing the NRD data. Dr. Almufti, Dr. Chebl, Dr. Miller, and Dr. Mayer contributed to scientific writing and project supervision. Dr. Mayer supervised the entire project, contributed to methodology, design, statistical planning, and manuscript writing. All authors contributed to data interpretation, composition and revision of the article. The manuscript was approved by all authors.