Cerebral Edema in Patients with severe Hemispheric Syndrome: Incidence, Risk Factors, and Outcomes—Data from SITS-ISTR
Article information
Abstract
Background and Purpose
Cerebral edema (CED) in ischemic stroke can worsen prognosis and about 70% of patients who develop severe CED die if treated conservatively. We aimed to describe incidence, risk factors and outcomes of CED in patients with extensive ischemia.
Methods
Oservational study based on Safe Implementation of Treatments in Stroke-International Stroke Treatment Registry (2003–2019). Severe hemispheric syndrome (SHS) at baseline and persistent SHS (pSHS) at 24 hours were defined as National Institutes of Health Stroke Score (NIHSS) >15. Outcomes were moderate/severe CED detected by neuroimaging, functional independence (modified Rankin Scale 0–2) and death at 90 days.
Results
Patients (n=8,560) presented with SHS and developed pSHS at 24 hours; 82.2% received intravenous thrombolysis (IVT), 10.5% IVT+thrombectomy, and 7.3% thrombectomy alone. Median age was 77 and NIHSS 21. Of 7,949 patients with CED data, 3,780 (47.6%) had any CED and 2,297 (28.9%) moderate/severe CED. In the multivariable analysis, age <50 years (relative risk [RR], 1.56), signs of acute infarct (RR, 1.29), hyperdense artery sign (RR, 1.39), blood glucose >128.5 mg/dL (RR, 1.21), and decreased level of consciousness (RR, 1.14) were associated with moderate/severe CED (for all P<0.05). Patients with moderate/severe CED had lower odds to achieve functional Independence (adjusted odds ratio [aOR], 0.35; 95% confidence interval [CI], 0.23 to 0.55) and higher odds of death at 90 days (aOR, 2.54; 95% CI, 2.14 to 3.02).
Conclusions
In patients with extensive ischemia, the most important predictors for moderate/ severe CED were age <50, high blood glucose, signs of acute infarct, hyperdense artery on baseline scans, and decreased level of consciousness. CED was associated with worse functional outcome and a higher risk of death at 3 months.
Introduction
Cerebral edema (CED) in acute ischemic stroke worsens the prognosis and can, if severe, cause life-threatening intracranial tissue shifts [1]. In patients presenting with cerebral ischemia, the size of the ensuing infarct is dependent on several factors, including treatment aimed at reperfusion of the occluded artery. Infarct size is a major determinant of the extent and clinical severity of the ensuing edema and potential hemorrhage. Severe hemispheric syndrome (SHS) is defined as having a high National Institutes of Health Stroke Score (NIHSS) score at baseline, as a marker of a large area of cerebral ischemia [2]. In cerebral ischemia caused by supratentorial large vessel occlusion (LVO), the risk of life-threatening edema is highest in occlusion of terminal internal carotid artery or the main trunk of the middle cerebral artery (MCA). This condition is called large hemispheric infarction (LHI). The most common guideline-supported definition of LHI refers to “a large ischemic stroke affecting the total or subtotal territory of the MCA, involving the basal ganglia at least partially, with or without involvement of the adjacent territories.” [3,4] Among patients with supratentorial ischemia, approximately 1 in 10 develops a subtotal or complete MCA infarction [2]. The clinical deterioration caused by edema after a large MCA infarction is usually observed within 48 hours after symptom onset with one third of the patients undergoing early deterioration within 24 hours [5]. The rate of mortality is close to 80% within a few days, unless treated with early surgery [6].
Data from previous studies indicate that the risk for life-threatening CED is strongly associated with increasing infarct size, or other variables correlated to infarct size such as need for mechanical ventilation, large perfusion deficit and involvement of more than one vascular territory [7-9]. A previous Safe Implementation of Treatments in Stroke (SITS)-International Stroke Treatment Registry (ISTR) paper reported that the most important baseline predictors for CED were higher NIHSS score, hyperdense artery sign (HAS), higher blood glucose, decreased level of consciousness (LOC) and signs of infarct at baseline imaging scan [10].
Aims and hypothesis
Although patients with SHS and LHI are at particular risk of CED, published data describing the burden and outcomes are limited. The aim of this study is to describe incidence, clinical characteristics, complications, and long-term outcome of CED in patients with SHS and persistent SHS (pSHS) after reperfusion treatment in a real-world setting population.
Methods
Study design
We included a subset of patients from the SITS-ISTR, an ongoing, prospective, internet-based, academic driven, multinational, primarily European Union-based, stroke register. Methods of data collection in SITS-ISTR have been described in detail elsewhere [10,11]. For this study, we have collected data from pre-specified intravenous thrombolysis (IVT) and or endovascular treatment (EVT) data entry forms of SITS-ISTR. All patients had presumed ischemic stroke treated with IVT and/or EVT and were recorded from January 2003 to September 2019. A study protocol and statistical analysis plan were developed before data extraction and analysis. According to the study protocol, we selected centers that included at least 10 patients in the SITS IVT and or EVT data entry forms and that had at least 70% complete data for modified Rankin Scale (mRS) score at 90 days follow-up.
The number of patients included in the study is not driven by a formal sample size calculation but rather by available patient numbers.
We included patients that:
• had a presumed ischemic stroke treated with IVT and/or EVT.
• were recorded at SITS-ISTR from January 2003 to September 2019.
• had a recorded NIHSS score at baseline.
• had a recorded indirect (HAS) or direct radiological evidence (computed tomography [CT] or magnetic resonance imaging angiography) of a cerebral arterial occlusion at baseline.
We defined the primary population study as patients with SHS at baseline (NIHSS score >15) and pSHS after reperfusion treatment (NIHSS score >15 at 24 hours).
Outcomes and covariates
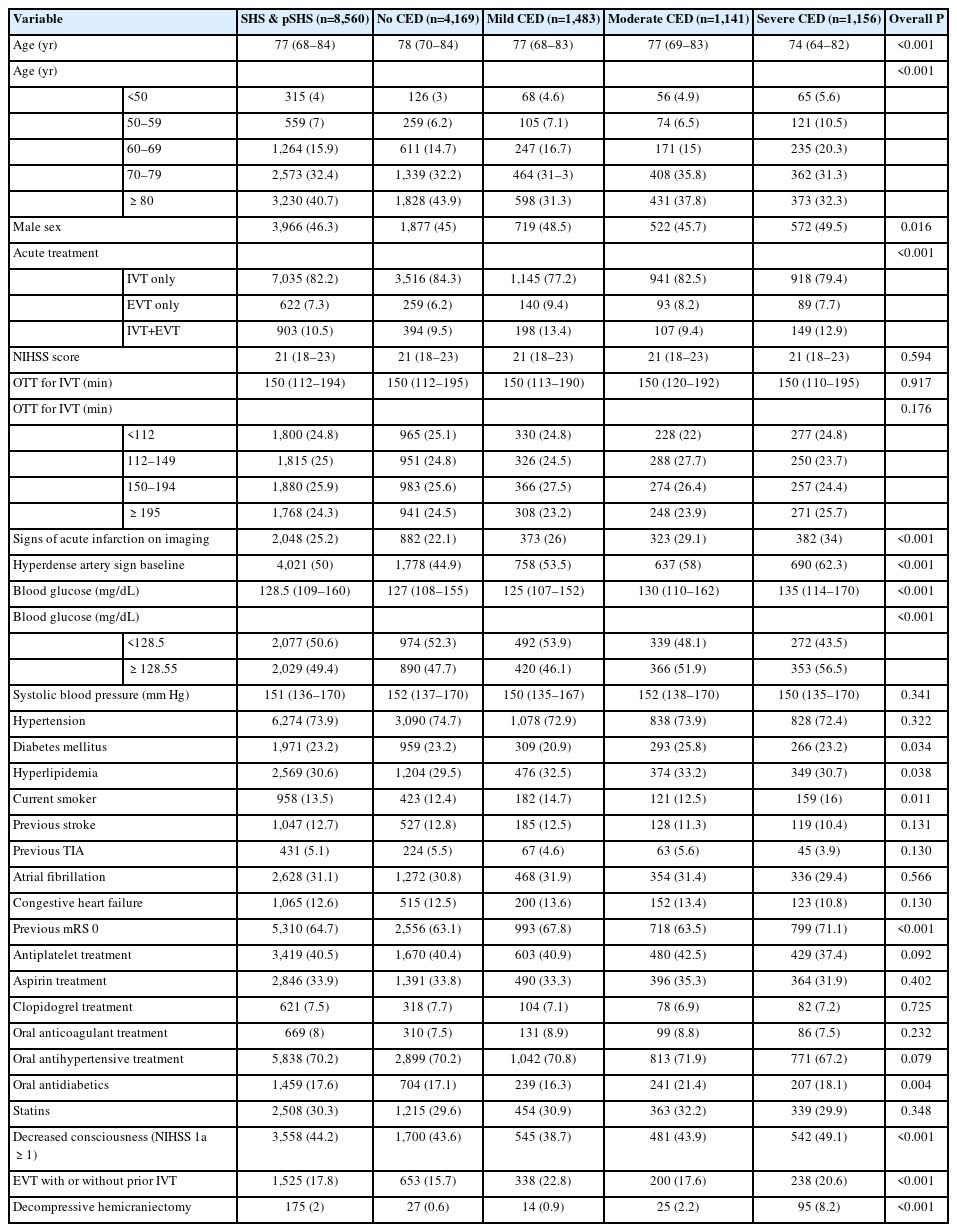
The primary outcome measure was CED which was rated by local investigators on post-treatment imaging scans at 22 to 36 hours. We used the SITS-Monitoring Study (MOST) edema scale which has been described previously [12]. We defined mild CED as focal brain swelling up to one-third of the hemisphere (grade 1), moderate CED as focal brain swelling greater than one-third of the hemisphere (grade 2), and severe CED as focal brain swelling with midline shift (grade 3). Although not explicitly mentioned in the SITS-MOST study protocol, signs of focal brain edema usually are defined as narrowing of the cerebrospinal fluid space, e.g., effacement of cortical sulci or ventricular compression. We specified in advance that the two higher grades of the scale would be put together into a compound outcome so that moderate to severe edema would be compared to no or mild edema.
Early secondary outcomes were the proportion of patients with parenchymal hemorrhage (PH) type 2 of the infarct area on follow-up imaging at 22 to 36 hours and the proportion of patients with symptomatic intracranial hemorrhage (SICH). To define SICH, we used the SITS-MOST (a local or remote type 2 PH <22 to 36 hours, or earlier in case of clinical deterioration, in combination with an increase of ≥4 NIHSS points from baseline, or leading to death <24 hours), and European-Australian Cooperative Acute Stroke Study 2 (ECASS-II) (any type of intracerebral hemorrhage with an increase of ≥4 NIHSS points from baseline or leading to death <7 days) definitions [11,13]. Late secondary outcomes, measured at 90 days, were mortality and functional outcome assessed by mRS score. Covariates collected for this study were baseline and demographic characteristics and any acute intervention.
Ethics and informed consent
Ethics approval was obtained from the Stockholm Regional Ethics Committee for this project as part of the SITS-MOST II study framework. Ethics approval and patient consent for participation in the SITS-ISTR were obtained in countries where required; other countries approved the register for anonymized audit.
Statistical analysis
In an initial univariate analysis, we compared baseline characteristics and outcomes between patients according to the presence or absence of CED, for all levels of CED and between the groups of no or mild edema versus moderate to severe edema. We used linear regression methods for numerical variables and Pearson´s chi-square test for categorical variables to compare baseline characteristics and outcomes. Estimation of proportions was based on reported cases, excluding unknown or uncertain values. A significance level of P<0.05 was used through the whole study.
We used multivariable regression analysis to identify predictors of CED. The biologically relevant baseline factors (NIHSS score, signs of acute infarction at baseline, HAS, blood glucose at baseline), and the factors associated (P<0.05) with CED in the previous univariate analysis were included in the multivariable model. Age was analyzed per 10-year groups and as a dichotomic variable (<50 years vs. ≥50 years). Association of baseline variables with CED were presented as relative risk (RR) with 95% confidence limits. A Poisson regression with generalized estimating equation was used to assess factors associated with CED. Impact of CED on long term outcomes was assessed using logistic regressions presented as an odds ratio. We used the statistical software SPSS version 25 (IBM Co., Armonk, NY, USA).
Results
Patients (n=8,560) that presented with SHS developed pSHS at 24 hours. Figure 1 shows a flowchart of the study. Table 1 shows baseline characteristics and univariate analysis of patients with SHS and pSHS, stratified by grade of CED. Median age was 77 and median NIHSS was 21 (interquartile range, 18 to 23); 46.3% were men. Of the total SHS and LHI patients, 7,035 patients (82.2%) received IVT, 903 (10.5%) IVT and EVT, and 622 (7.3%) EVT alone. Of 7,949 patients with available CED data, 3,780 (47.6%) had any CED and 2,297 (28.9%) were moderate/severe. Supplementary Table 1 shows baseline characteristics and Supplementary Table 2 shows the clinical and radiological outcomes of any SHS, any pSHS, both SHS & pSHS, and no SHS nor pSHS populations. Patients with pSHS had worse outcomes than SHS. pSHS patients had a higher rate of SICH and any type of hemorrhages and death and a lower rate of functional independence than SHS patients.
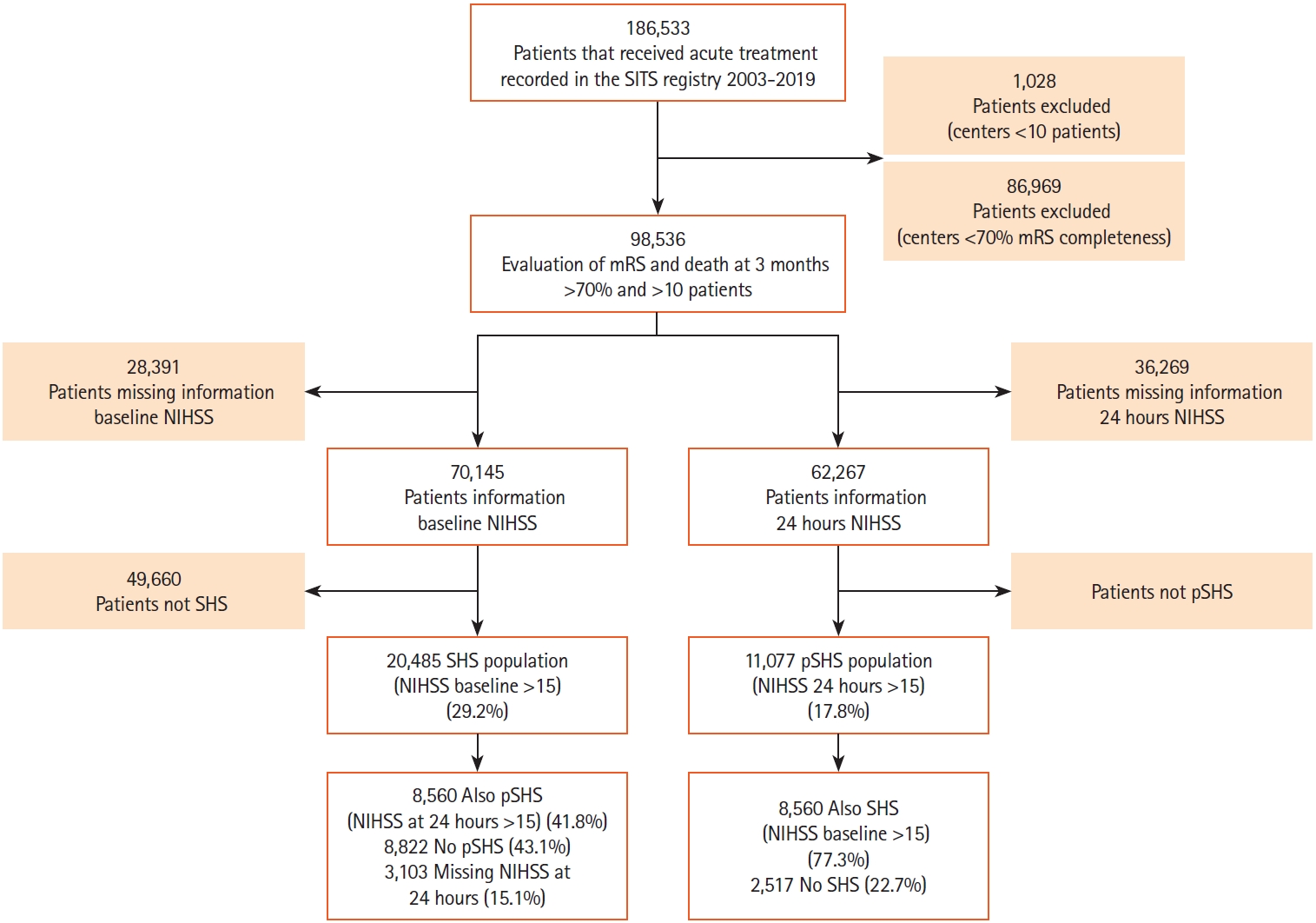
Study flow chart. SITS, Safe Implementation of Treatments in Stroke; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Score; SHS, severe hemispheric syndrome; pSHS, persistent severe hemispheric syndrome.
Univariate analysis
As seen in Tables 1 and 2, patients with moderate or severe CED were significantly younger and had more frequently signs of acute infarction and HAS at baseline imaging scans. Moreover, patients with moderate/severe edema presented higher values of blood glucose at admission and oral antidiabetic treatment. The proportion of patients with previous stroke was lower in the moderate/severe CED group and the percentage of patients with pre-stroke mRS 0 was higher. Decreased LOC as measured by item 1a in the NIHSS score was more frequent in the moderate/severe CED group. In 175 patients (2%) decompressive hemicraniectomy was performed. This percentage ranged from 0.6% in no CED, 0.9% in mild CED, 2.2% in moderate, and 8.2% in severe CED (P<0.001).
Multivariable analysis
Table 3 shows results from a multivariable model for prediction of moderate to severe CED with RR and 95% confidence intervals. Age <50 years (RR, 1.56), signs of acute infarction on baseline imaging (RR, 1.29), HAS (RR, 1.39), blood glucose >128.5 mg/dL (RR, 1.21), and decreased LOC (RR, 1.14) were associated with moderate/severe CED (for all P<0.05).
Long-term outcomes
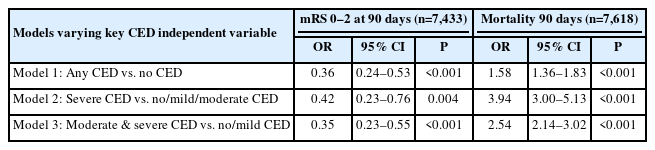
Table 4 shows multivariable logistic regression models for the effect of CED on long term outcomes. The adjusted odds ratio (aOR) showed that patients with any CED versus no CED (aOR, 0.36), severe CED versus no, mild, or moderate CED combined (aOR, 0.42), and moderate and severe CED versus no or mild CED (aOR, 0.35) were less likely to achieve functional independence defined by mRS 0–2 at 90 days. Regarding mortality, the presence of CED in any degree, moderate and severe and severe were associated with a higher risk for death at 90 days, and the risk increased with the severity of CED (aOR, 1.58 for any; aOR, 2.54 for moderate and severe; and aOR, 3.94 for severe).
Discussion
In this large observational study based on multinational data of acute ischemic stroke patients treated with recanalization therapy, we found that almost half of the patients with severe ischemic stroke according to SHS and pSHS definitions had any degree of CED and almost a third of them had moderate to severe CED. A previous Chinese study found the same rate of severe CED in LHI patients [14]. However, when all types of ischemic strokes irrespective of severity are analyzed, any type of CED, detected by the SITS-MOST Edema Scale [12] at 22 to 36 hours imaging was around one in four patients. The incidence of moderate and severe CED was similar, about 5% [10]. For the purpose of our study, we selected patients with severe stroke symptoms at baseline (SHS) and a clinical proxy using NIHSS score at 24 hours (pSHS) for severe strokes after repersfusion treatment. Our study population with SHS and pSHS are expected to have higher prevalence of CED than the general ischemic stroke population.
In this SHS & pSHS population with high risk of CED, the most important predictors for moderate/severe CED development were young age, high blood glucose levels, signs of acute infarction or HAS on baseline neuroimaging and decreased LOC at admission. Predictors for CED in our study on severe stroke patients are consistent with previous single center and multicenter studies that included broader populations. The strongest predictors for moderate/severe CED development were a younger age and HAS on baseline neuroimaging. These findings have been previously reported [10,12,15].
Elderly patients who developed large infarctions may have brain atrophy, and this may compensate for CED. HAS on baseline CT is an indirect finding of LVO and it is associated with SHS and LHI.
As previously reported, we also found that a higher blood glucose level at admission was a risk factor for CED development [10]. This consistent association between glucose levels and CED after a large ischemic stroke has been the basis of pre-and clinical research of glibenclamide as a potential treatment for these patients [16].
The association of recanalization therapies and CED remains unclear. In the line of our findings regarding signs of acute infarction at baseline, a recent meta-analysis has shown that in patients with LHI, EVT, and reperfusion were associated with severe CED only in the group with very large core volume [17].
Regarding long-term outcomes, the presence of any CED, moderate and severe and severe CED were independently associated with worse functional outcome, with a 60% decrease in the odds of functional independence. Our results regarding the effect of CED in functional outcome and mortality are consistent with a previous study in patients treated with IVT [9].
Presence of CED was associated with a higher risk of death at 90 days, including in-hospital mortality, and the risk increased from 1.5 to 4 times higher as compared to patients with no CED. Regarding treatment, randomized trials showed that surgical decompression reduced the risk of death, and meta-analyses of these trials showed that surgery also increased the chance of a favorable outcome [6,18].
Although being a well-known complication of large stroke, the management of CED remains a topic of discussion. The European Stroke Organization has recently published guidelines on the management of space-occupying brain infarction [19]. In this document, surgical decompression is recommended to reduce the risk of death and to increase the chance of a favorable outcome in adult patients aged up to and including 60years with space-occupying hemispheric infarction who can be treated within 48hours of stroke onset, and low-quality evidence to support this treatment in older patients. There is continued uncertainty about the benefit and risks of surgical decompression in patients with space-occupying hemispheric infarction if performed after the first 48hours. However, in a real-world setting, patients who undergo hemicraniectomy present frequently with complications that were underestimated in randomized clinical trials and may worsen the functional outcome, with 80% of survivors completely dependent (mRS 4–5) [20].
Recently, new guidelines on CED management in neurocritical care patients have been published [21]. For acute treatment, they suggest using either hypertonic sodium solutions or mannitol for the initial management of CED in patients with acute ischemic stroke (conditional recommendation, low-quality evidence), although neurological outcomes appear to be not affected. Previous guidelines state that there are insufficient data on the effect of hypothermia, barbiturates, or corticosteroids in the setting of ischemic cerebral swelling [3]. For these reasons, the management of patients with CED is still a challenge for stroke physicians, more clinical research is needed in early detection, prevention, and treatment of CED development.
This study presents some limitations. First, it is an observational study based on retrospective analyses, although data were collected prospectively. Our findings were limited to those who received IVT or EVT treatments and might not be generalizable to those who did not receive either therapy. Moreover, there is a proportion of missing and unknown data in some variables, including some missed cases of fatal CED, that may have influenced the results. Thus, there is a potential bias of patient selection. The associations observed in this study should be viewed as hypotheses-generating and need confirmation in further studies. Finally, another limitation is that we have defined persistent severe neurological deficit according to clinical criteria and not according to radiological findings, as data regarding final infarct volume were not available due to the lack of a centralized reading of the CTs. This could introduce some interobserver variability. However, pooled treatment of moderate/severe versus no/mild edema would tend to reduce this effect. The strengths of this study are the very large international sample size and consistent results with previous single and multicenter studies.
Conclusions
In conclusion, in patients with clinical signs of extensive ischemia, the most important predictors of moderate/severe CED development were young age, high blood glucose, signs of acute infarct, HAS on baseline scans and decreased LOC. The presence of any, moderate/severe, and severe CED were associated with worse functional outcome and a higher risk of death at 3 months, that increases proportionally with the severity of CED.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.01956.
Baseline characteristics of SHS, pSHS, SHS & pSHS, and no SHS nor pSHS populations
Clinical and radiological outcomes of the SHS, pSHS, SHS & pSHS, and no SHS nor pSHS populations
Notes
Disclosure
Niaz Ahmed is the Chairman of SITS International, which receives a grant from Boehringer Ingelheim for the SITS-International Stroke Thrombolysis Register and from Stryker, Covidien, and Phenox in collaboration with Karolinska Institutet for the SITS-OPEN study. Shih-Yin Chen is an employee of Biogen and owns stock in Biogen. Nicole Tsao was an employee of Biogen at the time of the study and owns stock in Biogen. This study was funded by Biogen. Other authors have no disclosures regarding conflict of interest.
Acknowledgements
SITS is financed directly and indirectly by grants from Karolinska Institutet, Stockholm County Council, the Swedish Heart- Lung Foundation, the Swedish Order of St. John, Friends of Karolinska Institutet, and private donors, as well as from an unrestricted sponsorship from Boehringer-Ingelheim. SITS has previously received grants from the European Union Framework 7, the European Union Public Health Authority, Ferrer International and EVER Pharma. SITS is currently conducting studies supported by Boehringer-Ingelheim and Biogen, as well as in collaboration with Karolinska Institutet, supported by Stryker, Covidien, and Phenox. Niaz Ahmed is supported by grants provided by the Stockholm County Council and the Swedish Heart-Lung Foundation. S Holmin is supported by grants provided by the Söderberg Foundations, the Stockholm County Council, the Erling Persson Foundation, VINNOVA, and HMT. Irene Escudero-Martínez has received a grant from “Fundación Progreso y Salud, Junta de Andalucía” (grant EF-0437- 2018). Magnus Thorén has received grants or funding from Stiftelsen Tornspiran, Stiftelsen Sigurd och Elsa Goljes Minne, STROKE-Riksförbundet, Capio forskningsstiftelse, and Karolinska University Hospital.