Trends in Stroke Presentations before and during the COVID-19 Pandemic: A Meta-Analysis
Article information
Abstract
Background and Purpose
There are reports of decline in the rates of acute emergency presentations during coronavirus disease 2019 (COVID-19) pandemic including stroke. We performed a meta-analysis of the impact of COVID-19 pandemic on rates of stroke presentations and on rates of reperfusion therapy.
Methods
Following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines, we systematically searched the literature for studies reporting changes in stroke presentations and treatment rates before and during the COVID-19 pandemic. Aggregated data were pooled using meta-analysis with random-effect models.
Results
We identified 37 observational studies (n=375,657). Pooled analysis showed decline in rates of all strokes (26.0%; 95% confidence interval [CI], 22.4 to 29.7) and its subtypes; ischemic (25.3%; 95% CI, 21.0 to 30.0), hemorrhagic (27.6%; 95% CI, 20.4 to 35.5), transient ischemic attacks (41.9%; 95% CI, 34.8 to 49.3), and stroke mimics (45.6%; 95% CI, 33.5 to 58.0) during months of pandemic compared with the pre-pandemic period. The decline was most evident for mild symptoms (40% mild vs. 25%–29% moderate/severe). Although rates of intravenous thrombolytic (IVT) and endovascular thrombectomy (EVT) decreased during pandemic, the likelihood of being treated with IVT and EVT did not differ between the two periods, both in primary and in comprehensive stroke centers (odds ratio [OR], 1.08; 95% CI, 0.94 to 1.24 and OR, 0.95; 95% CI, 0.83 to 1.09, respectively).
Conclusions
Rates of all strokes types decreased significantly during pandemic. It is of paramount importance that general population should be educated to seek medical care immediately for stroke-like symptoms during COVID-19 pandemic. Whether delay in initiation of secondary prevention would affect eventual stroke outcomes in the long run needs further study.
Introduction
Coronavirus disease 2019 (COVID-19) infection was initially reported from Wuhan, China in December 2019 [1]. It was declared a pandemic by World Health Organization in March 2020 [2]. There was a significant decrease in the hospital presentations and admissions, reported for most medical emergencies, including trauma, surgical emergencies, stroke, and acute coronary syndromes (ACSs) in regions with high numbers of COVID-19 cases [3-6]. A decrease in stroke admissions during the first peak of the pandemic was reported from Asia, Europe, North and South America [7-14]. While the decrease was predominantly recorded for those with milder symptoms, presentation for all stroke subtypes decreased substantially. This was suggested by decrease in the utilization of the computed tomography perfusion based rapid processing of perfusion and diffusion (RAPID, iSchemaView, Redwood City, CA, USA) software for acute stroke imaging in a report from USA [8,13,15-17].
The decrease in stroke admissions reported during the COVID-19 pandemic; however, has not been uniform, with conflicting reports from across the globe [18]. In addition, late presentation as reported by few has raised concerns that the pandemic may result in fewer patients receiving thrombolysis or endovascular thrombectomy (EVT) [19]. Several factors may have contributed to the recorded decrease in rates of stroke admissions and should be reviewed with caution [20-24]. Studies that provide information based on prospective registries or databases are more likely to offer accurate analysis of the changes developing during the pandemic. Reports comparing the change noticed during the pandemic to retrospectively collected pre-pandemic data tend to be less accurate and should be reviewed with caution. In view of above, a meta-analysis of the published reports may help establish the link between the impact of the COVID-19, rates of stroke admissions, rates of treatment with reperfusion therapy and likelihood of being treated with reperfusion therapy. We performed a systematic review and meta-analysis of observational studies during the COVID-19 pandemic between January 2020 and July 2021. We analyzed the data to answer following questions: (1) Was there a decrease in the rates of stroke hospitalization? (2) If a decrease in stroke rates was evident, was this specific to any particular stroke type (ischemic, hemorrhagic, transient ischemic attack [TIA]) and/or any National Institutes of Health Stroke Scale (NIHSS)-based severity (mild, moderate, severe)? (3) What was the effect of the pandemic on rates of thrombolysis and EVT?
Methods
Data sources and study selection
The data supporting the findings of study are available from the corresponding author upon reasonable request. The PubMed and Embase databases were systematically searched from January 1st, 2020, until July 24th, 2021, for studies published in English. We used a combination of the following terms for the database search: “Stroke,” “Cerebrovascular accidents,” “COVID-19,” “Coronavirus Disease 2019.” Details of the search strategy can be found in Supplementary Table 1. The current meta-analysis is compliant with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement [25].
Two authors (N.I. and A.J.B., both neurologists) independently screened the study titles and abstracts after removal of duplicates. Articles identified as potentially fulfilling our inclusion criteria underwent full-text evaluation by four authors (N.I., A.J.B., C.V., and R.N., all neurologists). We included studies only if they were original reports or observational studies with information on the rates of stroke cases and hospitalization before and during the COVID-19 pandemic. We excluded studies that did not provide information on the pre-COVID-19 stroke rates or were reviews without original data.
Data extractions and quality assessment
Publication quality was assessed using the Newcastle-Ottawa Quality assessment scale for cohort studies [26]. This scale is used to assess the Participant Selection, Comparability, and Outcome. A ‘good quality’ publication was defined as having 3 or 4 stars in selection domain and 1 or 2 stars in comparability domain and 2 or 3 stars in outcome domain. ‘Fair quality’ was defined as having 2 stars in selection domain and 1 or 2 stars in compatibility domain and 2 or 3 stars in outcome domain. ‘Poor quality’ was defined as having 0 or 1 star in selection domain or 0 stars in comparability domain or 0 or 1 stars in outcome domain. We only included studies that were of ‘good or fair quality.’
N.I., A.J.B., C.V., and R.N. extracted relevant data using a standardized data extraction form. Any disagreements were resolved by discussion. Extracted data included name of first author, year of publication, geographical location of the study, rate of total strokes, rate of ischemic strokes, hemorrhagic strokes, TIAs, stroke mimics, onset to door times (mean±standard deviation [SD]), classification of stroke center (primary or comprehensive stroke center), rates of reperfusion therapies, and severity of stroke based on NIHSS scores (mean±SD at presentation and number of mild [NIHSS <5], moderate [NIHSS of 5–15], and severe [NIHSS >15] strokes) before and during the pandemic. Wherever the mean±SD of NIHSS or onset-to-door time were not reported, they were estimated from the median and interquartile range [27]. Reperfusion therapy was defined as intravenous thrombolysis or EVT.
Statistical analysis
We used meta-analysis with random effects models to pool the percent change in the number of various stroke presentations (ischemic, hemorrhagic, TIA, stroke mimic, mild, moderate, severe) and the likelihood of receiving treatment with intravenous thrombolysis or thrombectomy across studies. For studies reporting the onset-to-door time, we pooled the standardized mean difference between the pre-pandemic and the pandemic period. Publication bias was assessed by inspecting funnels plots and performing the Egger test. Heterogeneity between studies and subgroups was assessed using the chi-square test on the Cochran Q statistic and quantified by the I2 index. All analyses were performed with STATA version 17.0 (StataCorp., College Station, TX, USA). All tests were 2-tailed and unpaired with a significance threshold of P≤0.05.
Results
The systematic database search retrieved 4,853 records, which were screened, and 116 studies underwent full-text evaluation. After excluding 79 studies for reasons outlined in Figure 1, 37 studies with 375,657 patients, meeting ‘fair or good’ quality criteria were selected for further analysis [8,28-63]. Twenty-four studies met criteria for ‘good’ quality and 13 met criteria for ‘fair’ quality (Supplemetary Tables 2-4). Most studies compared the pandemic period (ranging from January to June 2020) to a similar period in the preceding year (range January to June 2019) [28-31,33,35,39,41-43,45,46,48-50,52,54-58,60] or the months preceding the pandemic (range September 2019 to December 2019) [8,32,34,36-38,40,44,47,51,53,59,61-63].

Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of the observational studies selection process.
Rates of stroke admissions and severity of symptoms
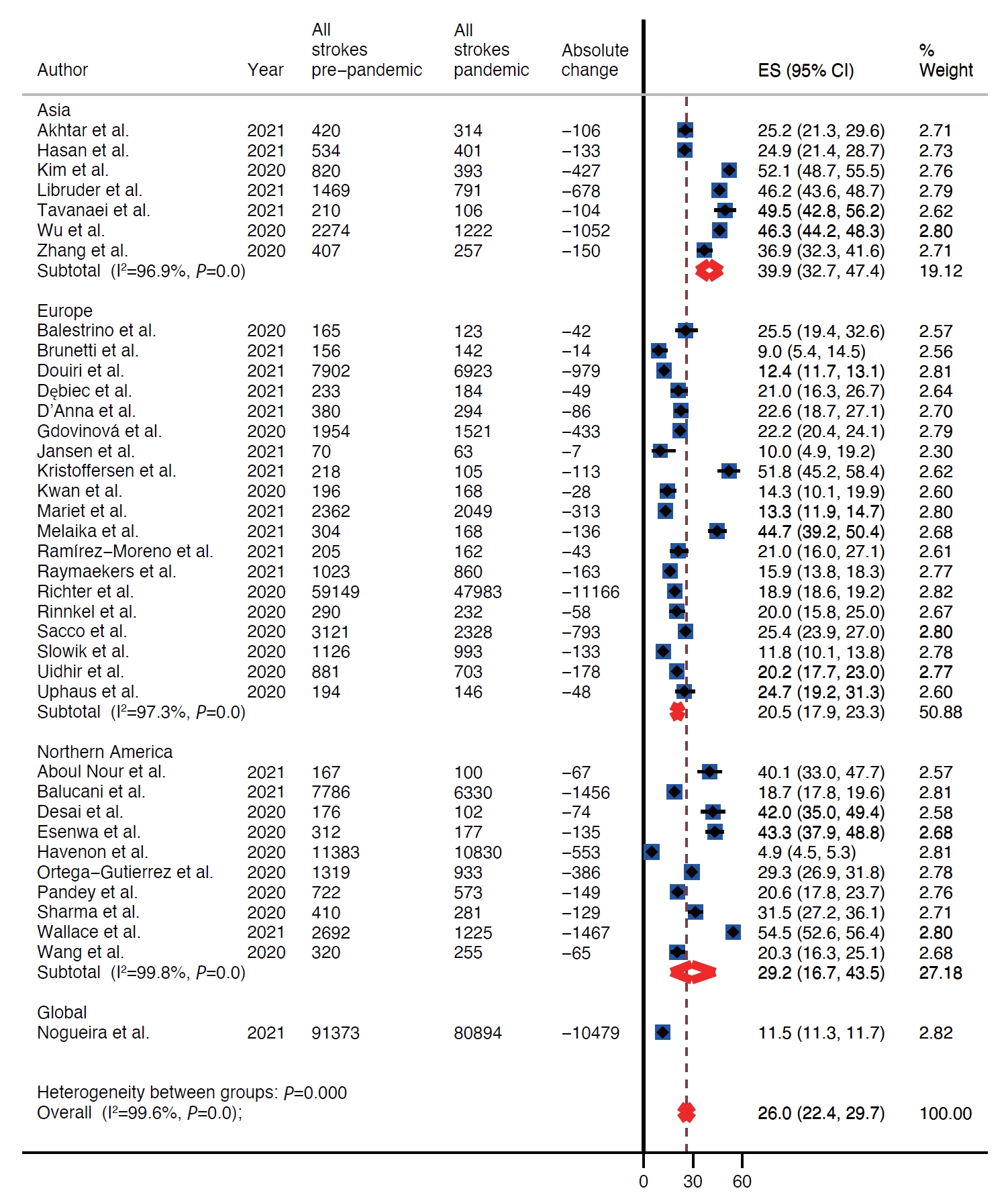
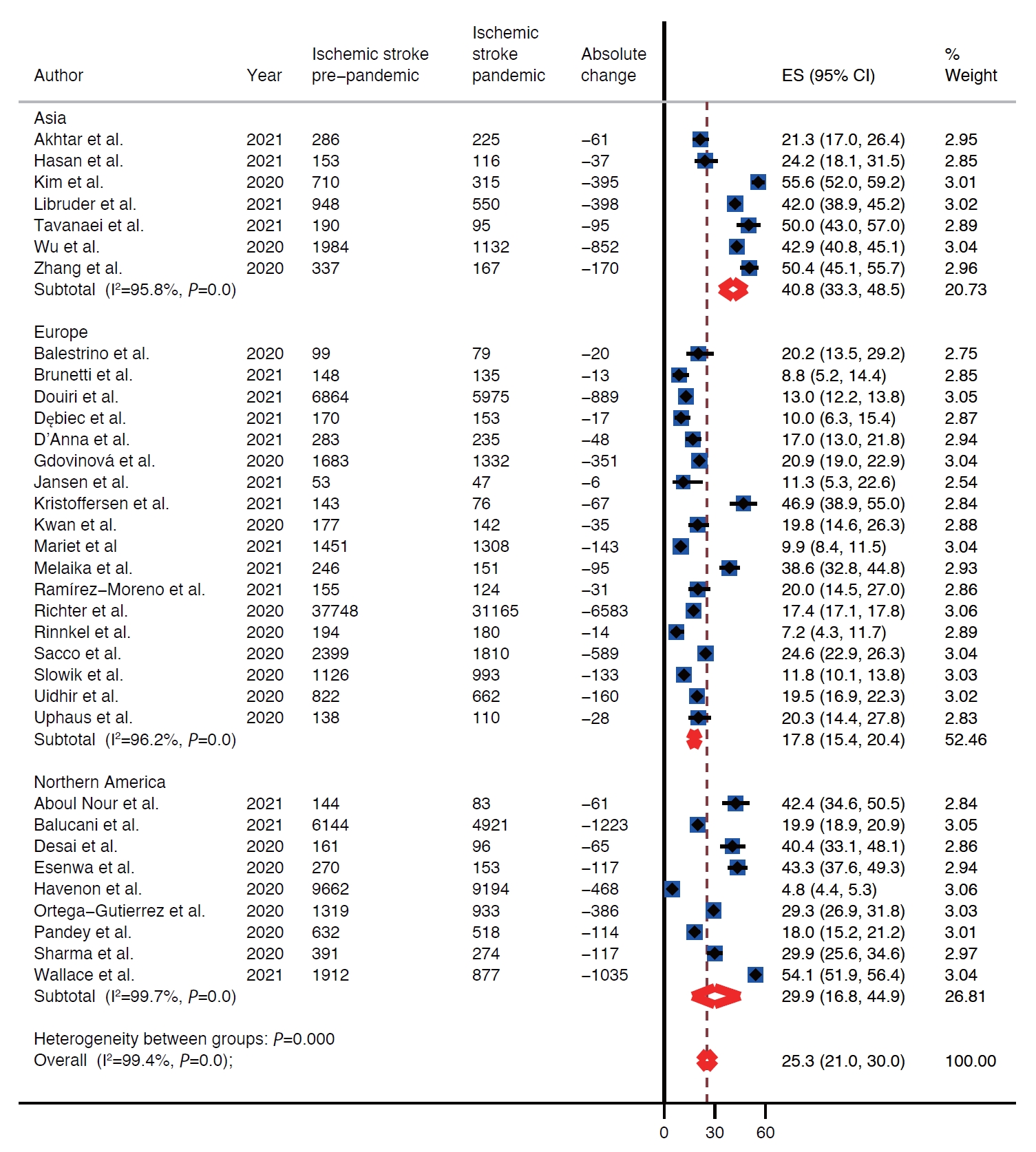
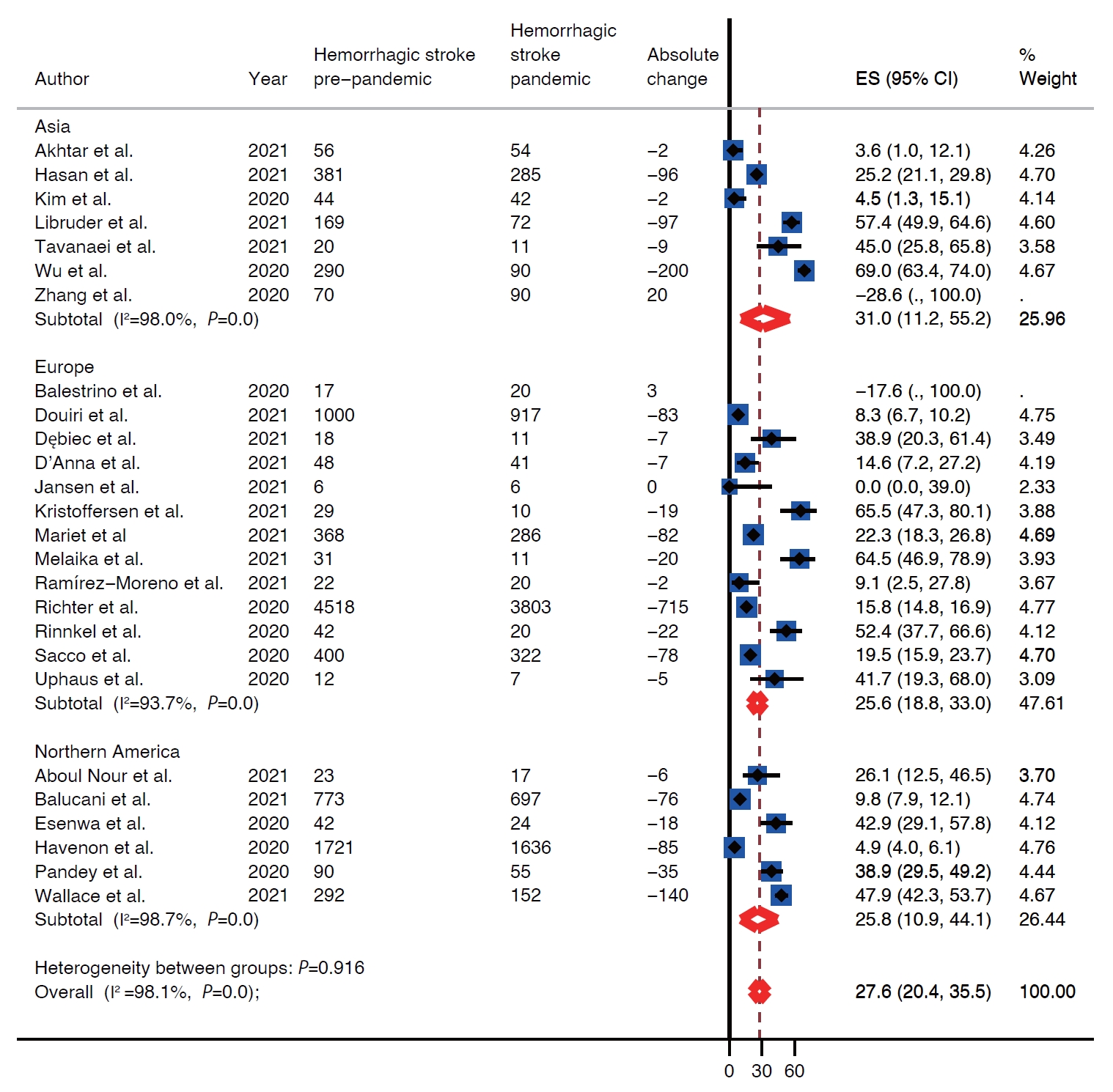
There was decline in rates of stroke admissions during the pandemic. The rate of all types of stroke presentations during pandemic was 26.0% lower than during pre-pandemic period (95% confidence interval [CI], 22.4 to 29.7) as shown in Table 1 and Figure 2. There was a publication bias with smaller studies reporting larger percent changes (Egger intercept=3.8; P=0.02) (Table 1 and Supplementary Figure 1). Analysis by stroke types showed that both ischemic and hemorrhagic stroke presentation rates decreased during the pandemic (Table 1). Specifically, the rate of ischemic stroke presentations during pandemic was 25.3% lower than during the pre-pandemic period (95% CI, 21.0 to 30.0) (Figure 3) while the rate of hemorrhagic stroke presentations during the pandemic was 27.6% lower (95% CI, 20.4 to 35.5) (Figure 4). Additionally, rates of TIAs and stroke mimics declined by 41.9% (95% CI, 34.8 to 49.3) (Supplementary Figure 2) and 45.6% (95% CI, 33.5 to 58.0) (Supplementary Figure 3), respectively.
Subgroup analysis revealed that there was a decrease in stroke rates during the pandemic for all severity categories with mild strokes (percent change, 40.2; 95% CI, 21.7 to 60.2) (Supplementary Figure 4) being the most affected (Table 1) as compared with moderate (percent change, 25.6; 95% CI, 11.0 to 43.8) (Supplementary Figure 5) and severe strokes (percent change, 29.1; 95% CI, 17.4 to 42.4) (Supplementary Figure 6). Regarding the distribution of mild, moderate, and severe strokes, there was an overall decrease in the share of mild strokes and a corresponding increase in the share of moderate and severe strokes (Supplementary Figures 7-9). During the pandemic, the odds for admitting a mild stroke versus a moderate or severe stroke was 0.78 (95% CI, 0.67 to 0.90; I2 =73.5%) (Supplementary Figure 7).
Regional difference
Included studies reported on rates of stroke presentations from Asia, Europe, and North America. The highest decrease in presentations for all types of strokes combined as well as ischemic strokes, hemorrhagic strokes, and TIAs during the pandemic was reported from Asia, 39.9% (95% CI, 32.7 to 47.4), 40.8% (95% CI, 33.3 to 48.5), 31.0% (95% CI, 11.2 to 55.2), and 51.5% (95% CI, 47.1 to 56.0), respectively (Table 1 and Figure 2). Whereas Europe had the smallest decrease in rates of presentations for all types of strokes combined, ischemic strokes, hemorrhagic strokes, and TIAs, 20.5% (95% CI, 17.9 to 23.3), 17.8% (95% CI, 15.4 to 20.4), 25.6% (95% CI, 18.8 to 33.0), and 38.3% (95% CI, 30.9 to 45.9) (Table 1 and Figure 2). Rates of presentations for all strokes combined, ischemic strokes, hemorrhagic strokes, and TIA decreased in North America by 29.2% (95% CI, 16.7 to 43.5), 29.9% (95% CI, 16.8 to 44.9), 25.8% (95% CI, 10.9 to 44.1), and 49.9% (95% CI, 17.5 to 82.3) (Table 1 and Figure 2). Highest decrease in rates of stroke mimics was reported from North America 78.1% (95% CI, 68.9 to 85.2) in comparison to 52.8% (95% CI, 47.5 to 58.1) in Asia and 39.7% (95% CI, 29.2 to 50.7) in Europe (Table 1 and Figure 2).
The admission rates of all strokes were reported to have dropped maximally during the pandemic in regions of the world that were most severely affected by the pandemic like certain states of the USA (California, Texas, New York, Illinois, Georgia, Ohio, Pennsylvania, New Jersey) [31,32,41,46], Italy [28,45], Iran [48], and Germany [50].
Time from onset to admission
As stroke treatment is time-sensitive, we next analyzed the time (in minutes) from onset/last seen well to hospital arrival. The onset-to-door time was reported in 14/37 studies [29,30,44,46,48,49,52,54,55,57-59,61,63]. There was no difference in mean onset-to-door time during pandemic when compared to pre-pandemic period (standardized mean difference=–0.2; 95% CI, –0.8 to 0.3).
Thrombolysis and endovascular treatment
The effect of the pandemic on the rates of thrombolysis was reported in 28/37 studies and 25/37 studies reported on the rates of EVT before and during the pandemic. The rate of intravenous thrombolytic (IVT) therapy for acute ischemic strokes dropped by 27.2% during the pandemic (95% CI, 22.7 to 32.0) (Supplementary Figure 10). This drop in rates of IVT was highest in Asia (40.3%; 95% CI, 27.8 to 53.3) followed by North America (26.9%; 95% CI, 12.7 to 43.9) and Europe (25.7%; 95% CI, 19.7 to 32.1) (Supplementary Figure 10). The likelihood of receiving IVT therapy did not differ between pre-pandemic and pandemic periods in primary stroke centers odds ratio (OR) 1.21 (95% CI, 0.93 to 1.57) as well as in comprehensive stroke centers OR 0.95 (95% CI, 0.83 to 1.09) (Figure 5). Although rates of EVT decreased during the pandemic by 20% (95% CI, 13.7 to 27.0) (Supplementary Figure 11), the likelihood of receiving EVT increased during the pandemic OR 1.11 (95% CI, 1.00 to 1.22) (Figure 6). Largest decrease in rates of EVT was in Asia 34.2% (95% CI, 19.4 to 50.7) followed by North America 20.7% (95% CI, 6.8 to 39.2) and Europe 15.6% (95% CI, 9.0 to 23.5). The likelihood of receiving EVT did not differ between two periods in comprehensive stroke center (OR, 1.08; 95% CI, 0.94 to 1.24) as well as in primary stroke center (OR, 1.54; 95% CI, 0.85 to 2.81) (Supplementary Figure 11).

Probability of receiving intravenous thrombolytic (IVT) based on type of stroke center. CI, confidence interval; REML, restricted maximum likelihood.
Discussion
In this systematic review and meta-analysis of 37 fair-to-good quality studies reporting the rates of stroke presentations in relation to the COVID-19 pandemic, we found that there was an overall significant decrease ranging between 25% and 50% in all stroke types including ischemic, hemorrhagic, TIAs, and stroke mimics during the months of the COVID-19 pandemic when compared with the pre-pandemic period. Stroke presentations declined nearly by approximately 40% for patients with mild symptoms. Although the absolute rates of IVT and EVT decreased during the pandemic, the likelihood of being treated with reperfusion therapy did not change during the pandemic either in primary or in comprehensive stroke centers. This systematic review and meta-analysis included some observational studies with publication bias but it is because of observational nature of studies and unique period of pandemic that might have affected data collection.
Among stroke categories, patients presenting with TIA had the highest decline during pandemic, with a decrease of 40%. Due to transient nature of neurological symptoms, patients may have chosen not to seek medical care as it might increase the risk of contracting COVID-19 infection. This trend is worrisome as it may lead to delay in diagnosis and initiation of prevention therapies. Patients with TIA are at higher risk of stroke in early period after TIA [14,21]. There is considerable evidence that the risk of stroke is reduced significantly with appropriate assessment and early treatment [21]. Whether delay in delivery of appropriate treatment will affect stroke outcomes subsequently is therefore a big concern. Population-based awareness campaigns to highlight the need to seek early medical attention should be conducted in the community, especially for those with TIAs and milder symptoms.
The rates of stroke mimics were significantly reduced by a percentage ranging from 33.5% to 58%. In the study from Qatar, a striking decrease to nearly one-thirds in rates of stroke mimic admissions was the major reason for the fall in stroke admissions during the pandemic months compared with the preceding months [8]. Patients with stroke mimics may avoid hospitalization due to fear of contracting COVID-19 infection [13,15,16,28].
Similar to TIAs, mild strokes decreased by 40% during the pandemic. In comparison, moderate strokes decreased by 25% and severe strokes decreased by 29%. Whereas, two recent systematic reviews and meta-analysis on stroke in patients with COVID-19 infections revealed that stroke is an uncommon complication of the illness and develops in less than 1.5% of patients [64,65]. Interestingly, initial reports also suggested that strokes of increased severity were seen more frequently in patients with severe COVID-19 infections admitted to hospitals [7]. These cases may be secondary to the direct prothrombotic effects of the COVID-19 illness. There are reports of the formation of recurrent thrombi during the treatment of acute stroke [7]. The COVID-19 virus may directly damage the cerebral vascular endothelium, making it more prothrombotic and this may explain the higher incidence of severe strokes [8,9,66-69].
While different trends were observed for thrombolysis delivery in various studies across the globe, our composite analysis shows that the rates of both IVT and EVT dropped by slightly more than one-fourth and one-fifth during the pandemic. This may be related to possible delayed hospital arrival and an overall decrease in the absolute number of patients with mild and moderate stroke seeking medical care [21,29,30,46,54,55,59,60,62,70]. However, the likelihood of being treated with IVT did not differ between two periods in comprehensive stroke centers and that of being treated with EVT increased during the pandemic, which might be due to adequate changes made in workflow of acute stroke care in comprehensive stroke centers [71]. Higher likelihood of being treated with EVT might also have been caused by higher likelihood of large vessel occlusions due to prothrombotic state driven by COVID-19 virus, as reported by multiple studies [7-9,66-69].
There was a decrease in the rates for all types of stroke from all geographical regions of the world. These findings are similar to the decrease in admission rates of several other illnesses [4-6]. A decrease in admission rates for ACS has been reported from all geographic regions of the world and appears to parallel the severity of the lockdowns [72]. A decrease for most acute and chronic illnesses has also been reported from New York recently, where the effect was most apparent for infections and septicemia [73]. In Qatar, a decline in admissions to the emergency department varying from 9% to 75% was observed for acute surgical emergencies, ACS, bone fractures, and cancer whereas admissions for respiratory conditions increased [5]. In Finland, there was a reduction in the rates of several acute medical illnesses seen in the emergency department, including infections (28%), back or limb pain (31%), and psychiatric illness (19%). Interestingly and in contrast, there was no decrease in the number of stroke or ACS admissions during the period of observation [21]. This may be driven by selection bias due to severity of stroke symptoms or reporting of acutely managed cases.
The most prevalent hypothesis for decline in rates of presentation of acute illnesses to the hospital relates to the fear of contracting COVID-19 when coming to the hospital. It may stand especially true for patients with transient or milder symptoms [20,21,40]. This in turn may be magnified from ‘stay-athome’ orders, leading to deferring urgent care as suggested in a recent survey from the United Kingdom [74]. In Germany, the initial early decline in stroke-related consultations in the pandemic and later increase for telemedicine services, paralleled the population activities during lockdowns [22]. Another study from France also reported that there appeared to be a relationship between the decrease in stroke admissions and the severity of the COVID-19 pandemic [60]. The alternative hypothesis is that of decreased incidence of cardiovascular events related to lifestyle changes [40,75]. Similarly, in Greece the significant decrease in ACS admissions in three municipalities appeared to be directly related to lifestyle changes including reduced passive smoking, working hours, alcohol and junk food consumption, and increased sleeping hours related to lockdown [76]. Although appealing, the study mainly addressed people with low burden of cardiovascular risk factors and thus the results should be interpreted carefully.
Other factors proposed to explain the decrease in emergency visits for acute illnesses include reduced social contact resulting in lowered “third-party” detection of unappreciated acute stroke symptoms [40]. Another speculation is that of beneficial reduction in air pollution related to decreased carbon dioxide emissions and lower temperatures in relation to lockdowns during the peak of the pandemic [21,22,77]. A decrease in physical activity during lockdown may also have potential protective effects. An increase in physical activity is known to increase blood pressure, potentially increasing the risk of stroke and ACS [20,78].
Our study has certain strengths and limitations. First, it is a composite analysis of studies comparing pre-pandemic to pandemic period and thus addresses the skepticism around the commonly raised concerns regarding stroke care. Second, we not only compared stroke presentations, but also analyzed the effect of stroke severity on relative differences in presentations. Third, the results are based on studies from multiple continents and diverse regions which is reflective of the global impact of the pandemic. The main limitation of this analysis is that this was based on observational studies. Also, although the likelihood of thrombolysis and thrombectomy seems unchanged, the effects the pandemic might have on stroke outcomes in terms of secondary prevention warrants further study. Comprehensive prospective registries recording the above stated parameter may help address these concerns as the pandemic evolves.
Conclusions
We meta-analyzed 37 studies that reported the rates of stroke presentation before and during the COVID-19 pandemic from various geographic regions. Rates of all stroke types declined significantly during the pandemic, but most profoundly for transient and milder symptoms, and stroke mimics. This resulted in lower rates of treatments with IVT as well as EVT. Whether delay in delivery of secondary prevention for those with mild symptoms would affect eventual stroke outcomes in the long run needs further study.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2021.01571.
Search strategy
Characteristics of the included studies
Characteristics of included studies based on stroke severity, IVT, and EVT
MOOSE statement: reporting checklist for authors, editors, and reviewers of meta-analyses of observational studies
Funnel plot for the meta-analysis of the percent changes in the number of all-type strokes.
Percent change in the number of transient ischemic attacks. TIA, transient ischemic attack; ES, effect size; CI, confidence interval. *This statistics could not be computed due to small number of studies (n≤3).
Percent change in the number of stroke mimics. ES, effect size; CI, confidence interval. *This statistics could not be computed due to small number of studies (n≤3).
Percent change in the number of mild strokes (National Institutes of Health Stroke Scale [NIHSS] <5). ES, effect size; CI, confidence interval. *This statistics could not be computed due to small number of studies (n≤3).
Percent change in the number of moderate strokes (National Institutes of Health Stroke Scale [NIHSS] 5–15). ES, effect size; CI, confidence interval. *This statistics could not be computed due to small number of studies (n≤3).
Percent change in the number of severe strokes (National Institutes of Health Stroke Scale [NIHSS] >15). ES, effect size; CI, confidence interval. *This statistics could not be computed due to small number of studies (n≤3).
Probability of having a mild stroke (National Institutes of Health Stroke Scale [NIHSS] <5). CI, confidence interval.
Probability of having a moderate stroke (National Institutes of Health Stroke Scale [NIHSS] 5–15). CI, confidence interval.
Probability of having a severe stroke (National Institutes of Health Stroke Scale [NIHSS] >15). CI, confidence interval.
Percent change in the total number of intravenous thrombolysis (IVT) performed. ES, effect size; CI, confidence interval.
Percent change in the total number of endovascular thrombectomies (EVTs) performed. ES, effect size; CI, confidence interval.
Notes
Disclosure
The authors have no financial conflicts of interest.