Early Life Body Size in Relation to First Intracerebral or Subarachnoid Hemorrhage
Article information
Abstract
Background and Purpose
As risk of hemorrhagic stroke may have early life origins, we investigated associations of birth weight and childhood body mass index (BMI) with adult intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH).
Methods
We included 240,234 Danish schoolchildren, born 1936 to 1989, with information on birth weight and measured weights and heights from 7 to 13 years. We calculated hazard ratios (HRs) and confidence intervals (CIs) for the associations between early life anthropometrics and ICH or SAH, identified through linkage with national registers.
Results
During the study period, 1,947 individuals (39% women) experienced an ICH and 797 individuals (64% women) experienced a SAH. Per 500 g increase in birth weight, women had a 10% decreased risk of SAH (HR, 0.90; 95% CI, 0.83 to 0.97) and men had a 10% decreased risk of ICH (HR, 0.90; 95% CI, 0.85 to 0.95). Birth weight was not associated with risks of ICH in women or SAH in men. In men, a childhood BMI below average (BMI z-score <0) was associated with increased risks of ICH. The association was stronger at older childhood ages, and at 13 years a BMI z-score of –1 was associated with a HR of 1.17 (95% CI, 1.06 to 1.28), and a BMI z-score of –2 with a HR of 1.46 (95% CI, 1.17 to 1.82) for ICH. Childhood BMI was not associated with risks of ICH in women or with risks of SAH in both sexes.
Conclusions
Early life body size is associated with ICH and SAH, and the associations differ by sex.
Introduction
While the incidence of hemorrhagic stroke has decreased worldwide, an increase in incidence has been observed in lowand middle-income countries [1], and intracerebral hemorrhages (ICHs) and subarachnoid hemorrhages (SAHs) are still the dominating causes of stroke-related deaths worldwide [2]. More men than women are diagnosed with an ICH, whereas the opposite is the case for SAH. Moreover, in contrast to ischemic stroke, ICH and SAH often occur at younger adult ages [1]. The subdivision of hemorrhagic stroke is primarily based on the location of the bleeding, but the etiology of ICH and SAH also differs. An ICH is typically a result of small vessel disease due to chronic hypertension, which causes micro-aneurysms that subsequently rupture [3,4], whereas ruptured larger aneurysms usually cause SAH [3]. It is therefore likely that these two hemorrhagic stroke subtypes also have different relationships with early life risk factors and that these relations may differ by sex.
Low birth weight has been identified as a risk factor for ischemic stroke, but most studies do not distinguish between the major stroke subtypes so the results may be different for ICH and SAH because of the difference in aetiology [5]. Studies investigating the influence of childhood body mass index (BMI) on stroke are limited by low numbers of stroke cases thus restricting their possibilities of conducting sex-specific analyses and investigating stroke subtypes [6-10]. This may be why childhood BMI is not yet included as a risk factor in itself in the stroke prevention guidelines like birth weight is. We recently found that having a BMI above average at 7 to 13 years or having an above average gain in BMI in that period of childhood increases the risk of experiencing an ischemic stroke in early adulthood (before age 55 years) in women and men [11]. Another recent study reported positive associations of gain in BMI from 8 to 20 years, but not with childhood BMI in itself, with risks of ischemic stroke as well as with ICH among Swedish men [10]. However, apart from only including men, this study did not investigate effects of birth weight nor did it include SAH as an outcome.
We investigated associations of birth weight, childhood BMI and change in BMI from 7 to 13 years with later risk of ICH or SAH separately for women and men, in a cohort of Danish schoolchildren born from 1936 to 1989.
Methods
We used the computerized Copenhagen School Health Records Register (CSHRR) [12] with information on birth weight and annually measured weights and heights of 372,636 children, who were born between 1930 and 1989. The register contains virtually every child who attended a public or private school in the Copenhagen Municipality. The children were examined during their school years (7 to 13 years) by trained school physicians and nurses using standard procedures. Birth weight, as reported by the parents at the first school health visit, was available for children born in 1936 and onwards.
Since 1968, all Danish citizens have been assigned a unique identification number, and by using this number we were able to link the information contained in the CSHRR with the Danish National Patient Registry, which contains all hospital discharge diagnosis since 1977 [13], and with the computerized Danish Cause of Death Register, which was established in 1970 [14].
We identified stroke events by using the International Classification of Disease (ICD) System. In Denmark the 8th revision was used until 1994, and the 10th revision was used thereafter. All first-ever diagnoses of non-traumatic ICH (ICD-8: 431; ICD-10: I61) and non-traumatic SAH (ICD-8: 430; ICD-10: I60) were retrieved for the individuals included in the CSHRR.
We followed the children from 1977, when the Danish National Patient Registry was established, or from age 25 years, until the first ICH or SAH event, loss to follow-up, emigration, death by another cause or 31st December 2015. The analyses were conducted on anonymous data, and the study was approved by the Danish Data Protection Agency. According to Danish law, ethical approval or informed consent is not required for purely register-based research.
Statistical analysis
Children with extreme values of birth weight (<2.0 or >5.5 kg) were excluded from the analyses to avoid including children with low birth weight due to prematurity, as well as children of diabetic mothers, which may be overrepresented in very high birth weight group. BMI was calculated (kg/m2) and transformed into z-scores based on an internal reference of children born between 1955 and 1959, where the prevalence of obesity was low and stable in Denmark.
We used Cox proportional hazards regression, with age as the underlying time axis, to investigate associations of birth weight and childhood BMI with ICH or SAH separately among women and men. We investigated the shape of the associations by using a categorical model and a restricted cubic spline model with 3 knots. Linearity was investigated by testing the linear model against these two models, using the likelihood ratio test. We tested the proportional hazards assumption by comparing the associations within quartiles of age. Potential interactions between birth weight and BMI in the association with ICH or SAH were investigated using the likelihood ratio test. When investigating change in BMI from 7 to 13 years we divided BMI into four groups at age 7 and 13 years based on the International Obesity Task Force (IOTF) cut-offs from 2012 [15] for thinness, normal weight, overweight and obesity (corresponding to BMI<18.5, 18.5 to 25, >25 to 30, and >30 kg/m2 at age 18 years) and compared children who decreased or increased in BMI category between 7 and 13 years with the reference group of children who stayed in the normal weight category. Because major societal changes as well as changes in treatment and management of stroke and stroke risk factors occurred during the study period, all analysis were stratified by birth cohorts (5-year intervals) and we also formally investigated if the associations differed by birth cohort by the likelihood ratio test.
As imaging by computed tomography and magnetic resonance imaging was implemented during the years included in the study, we conducted a sensitivity analysis investigating the associations among those who were diagnosed during the last 10 years of follow-up, and who therefore were likely to have a diagnosis based on imaging.
Results
The study population consisted of 240,234 women and men included in the CSHRR with information on birth weight and at least one measure of childhood BMI (Figure 1). During the years of follow-up (mean follow-up time, 28.2 years) 762 women and 1,185 men were diagnosed with an ICH, and 507 women and 290 men were diagnosed with SAH. The incidence rate of ICH increased with age, but more steeply in men, whereas the incidence rate for SAH seemed to be highest in the middle-aged years in both sexes (Figure 2). Small differences in birth weight and childhood BMI were observed between those who did not experience any hemorrhagic stroke later in life versus those who did (Table 1).
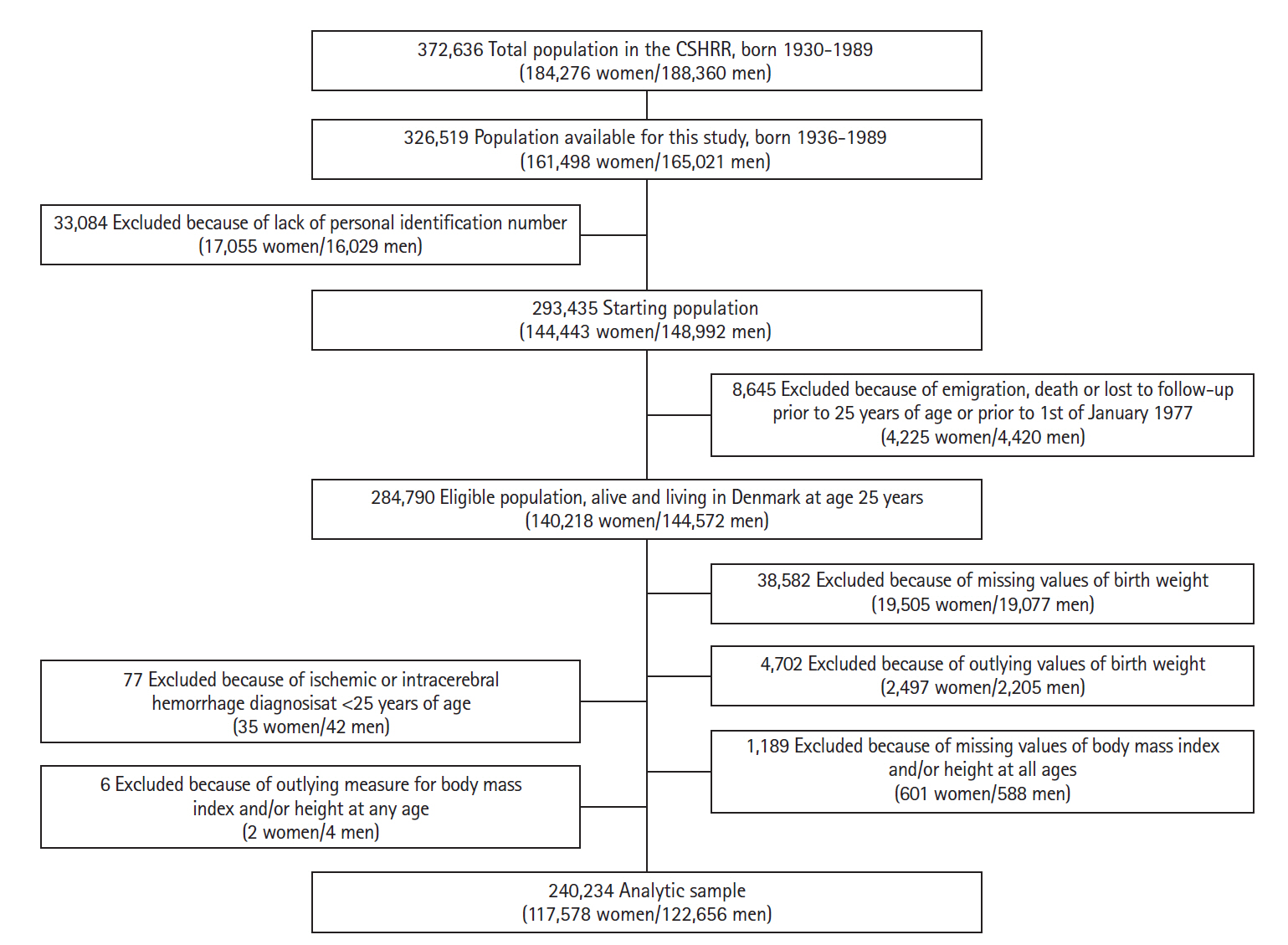
Flowchart describing the criteria of eligibility in the overall study (n=240,234), based on data from the Copenhagen School Health Records Register (CSHRR).

Incidence rate of adult hemorrhagic stroke (cases per 1,000 person-years) for each subtype according to age at diagnosis. (A) Intracerebral hemorrhage. (B) Subarachnoid hemorrhage.
Intracerebral hemorrhage
We found indications of different associations of birth weight with risks of ICH in women compared with men (likelihood ratio test for sex-interactions, P=0.02). Lower birth weight was not associated with ICH among women but was in men for whom a 500 g increase in birth weight was associated with a 10% decrease in the risk of ICH (Figure 3).
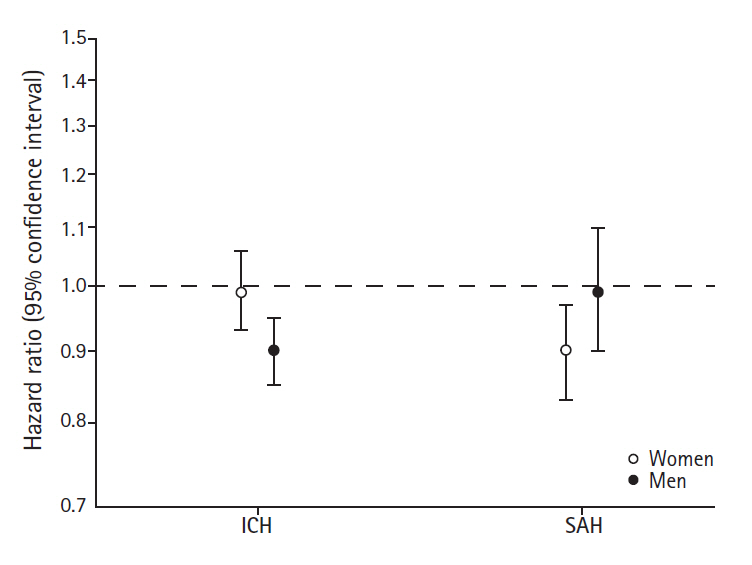
Risks of intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH) per 500 g increase in birth weight among women and men.
For of the associations between childhood BMI and later ICH we found a similar pattern at all childhood ages, and therefore only present results for age 7 and 13 years (Figure 4, other ages are presented in Supplementary Figure 1). Among women, we did not find that childhood BMI was associated with later risks of ICH. Among men, however, we found a non-linear pattern, where men who had a childhood BMI below average (BMI z-score below 0), had increased risks of ICH. The risk of ICH was higher among those with lowest BMI z-scores and the associations were stronger at older ages in childhood. At age 13 years, having a BMI z-score of -2 was associated with a hazard ratio (HR) of 1.46 (95% confidence interval [CI], 1.17 to 1.82), and a BMI z-score of -1 was associated with a HR of 1.17 (95% CI, 1.06 to 1.28) for ICH. We found no interactions with birth weight in the association between childhood BMI and ICH (likelihood ratio test, all P>0.20). Further, as illustrated in Figure 4, adjusting the childhood BMI-ICH results for birth weight only minimally affected the associations between childhood BMI and ICH.
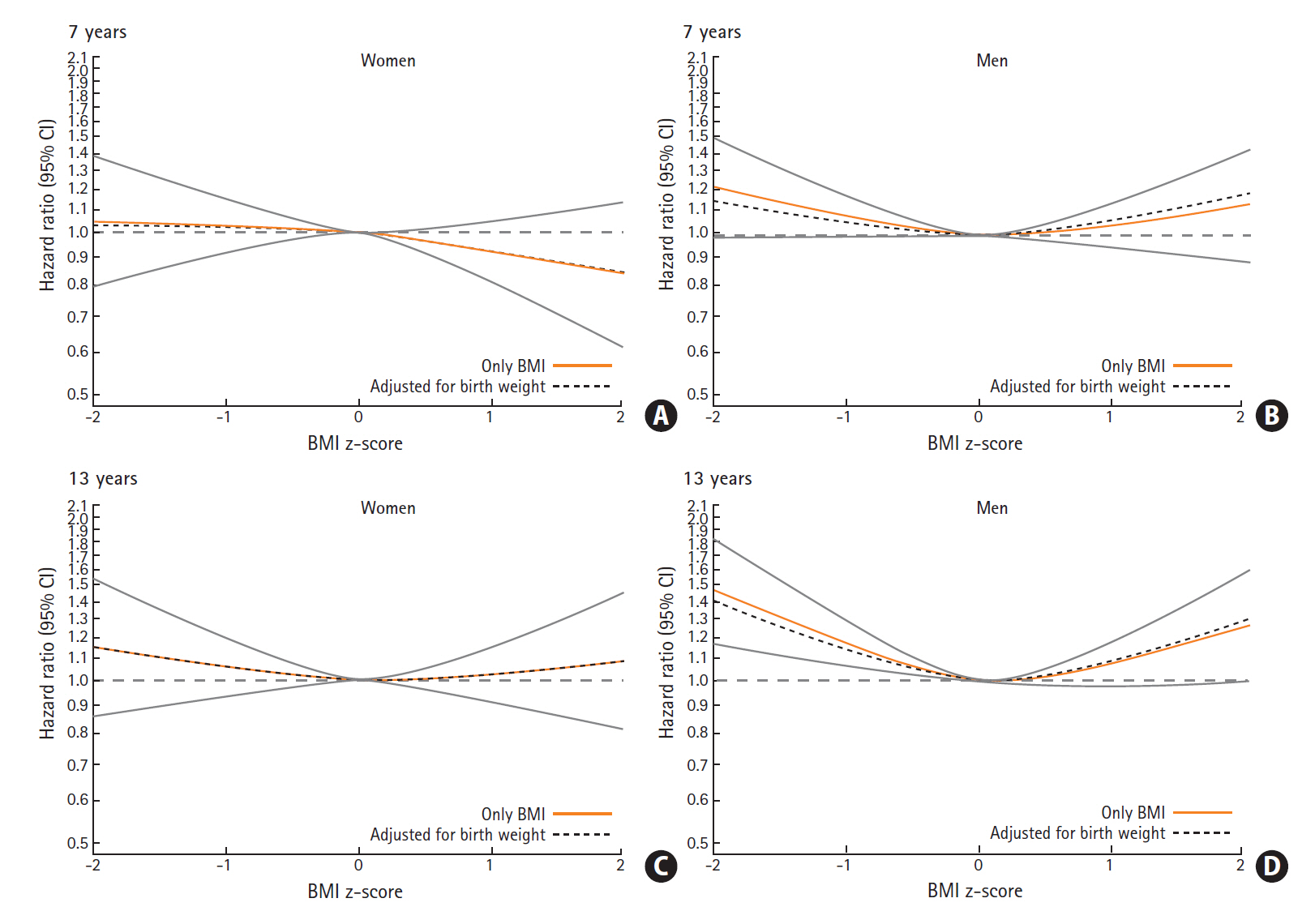
Hazard ratios (HRs) with confidence intervals (CIs) for the association between childhood body mass index (BMI) at age 7 and 13 years and risks of intracerebral hemorrhage among women and men. (A) Women, 7 years. (B) Men, 7 years. (C) Women, 13 years. (D) Men, 13 years.
Change in BMI from 7 to 13 years was not associated with risks of ICH among women, but was in men (Table 2). Men who were defined as normal-weight at age 7 but thin at age 13 years also had increased risks of ICH (HR, 1.33; 95% CI, 1.05 to 1.70), whereas men defined as thin according to the IOTF cutoffs at both ages 7 and 13 years only showed a tendency to an increased risk of ICH (HR, 1.19; 95% CI, 0.90 to 1.84).

Change in childhood BMI according to IOTF category between 7 and 13 years and risks of ICH among women and men
None of the associations differed according to age at diagnosis (all P>0.05, except for BMI at 10 and 13 years among boys, with P-values of 0.04, when investigating potential interactions between quartiles of age at diagnosis using the likelihood ratio test), or according to birth cohort (all P>0.20 when investigating potential interactions between birth cohorts using the likelihood ratio test).
When restricting the analysis to only cases of ICH diagnosed within the last 10 years, we found similar results (Supplementary Table 1 and Supplementary Figure 2).
Subarachnoid hemorrhage
For SAH, we found that women who were small at birth had an increased risk; a 500 g increase in birth weight was associated with a 10% decreased risk of SAH. Among men birth weight was not associated with SAH (Figure 3). The sex-difference in the associations was however not statistically significant (likelihood ratio test for sex-interactions, P=0.10).
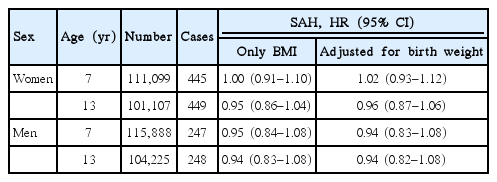
Childhood BMI at any age was not associated with SAH in women or in men, and therefore only results for age 7 and 13 years are presented (Table 3, other ages are presented in Supplementary Table 2). Moreover, we did not detect any interactions between birth weight and BMI in the association with SAH (likelihood ratio test, all P>0.20), and adjusting the childhood BMI-SAH associations for birth weight did not change the findings (Table 3).
For this hemorrhagic stroke subtype, the number of cases in the different BMI-strata was too low to provide reliable estimates of risks. We did not find that the associations differed according to age at diagnosis or according to birth cohort (all P>0.10 or P>0.20, respectively, when using the likelihood ratio test).
The sensitivity analysis based on cases SAH diagnosed within the last 10 years revealed similar results (Supplementary Tables 1 and 3).
Discussion
In this large population-based cohort of Danish schoolchildren, we found that lower birth weight was associated with increased risks of SAH in women and with increased risks of ICH in men. In women childhood BMI was not associated with risks of ICH, whereas men who had a BMI below average during the school years had increased risks of ICH. No associations between childhood BMI and SAH in either sex were found, and change in BMI during childhood was not related in itself with ICH in women, but in men those who became thin during childhood had increased risks of ICH.
In contrast to prior studies, we identified sex-differences in the associations between birth weight and risks of hemorrhagic stroke according to subtype. In the Helsinki Birth Cohort, birth weight was inversely associated with increased risks of ICH and SAH combined, but only when adjusting for head circumference [8,9]. This and our findings are in accordance with what was found in the Nurses’ Health Study, where a lack of an association between birth weight and hemorrhagic stroke among women was reported [16]. However, in the Aberdeen Children of the 1950’s study and in a cohort from Uppsala strong inverse associations of birth weight with ICH and SAH in women and men combined were observed [17,18]. Nonetheless, in an updated analysis on the Uppsala cohort this association disappeared when women and men were examined separately even though almost 30% of the cohort participants had a stroke [19].
Surprisingly, we found that a childhood BMI below average was associated with increased risks of ICH among men, and we also found that changing from being classified as normal weight to thin from 7 to 13 years was associated with increased risks of ICH among men. These findings contrast with what a study on Swedish men that included 207 cases of ICH showed; where childhood BMI was not associated with ICH, but gain in BMI from 8 to 20 years and adult BMI were positively associated with ICH [10]. One of the studies based on the Helsinki Birth Cohort reported that the findings of an association between lower BMI at age 7 years, but not at age 11 years, and total stroke, were similar for ischemic and hemorrhagic stroke (i.e., ICH and SAH combined) [9]. Generally, in adults both low and very high BMI are suggested to have associations with hemorrhagic stroke, but the studies that have investigated this have mainly been focused on effects of high BMI [5].
Studies in the United Kingdom identified low birth weight and normal head circumference as a risk factor for stroke mortality [20]. They suggested that this was due to late disrupted gestational growth, as head growth occurs relatively early in gestation. The replication of these findings in the Helsinki Birth Cohort, however, led to the hypothesis that stroke originates through patterns of reduced fetal growth in which the brain is spared, and this phenomenon was termed brain-sparing [8]. The authors further speculated that the redistribution of cardiac output in favor of the brain may cause permanent adverse changes of the structure of the major arteries in the truncus and brain. This may as well as compromise the development of the abdominal organs, including the kidneys, which later in life could lead to hypertension, the most important risk factor for hemorrhagic stroke.
Low birth weight has previously been linked with increased risks of later hypertension in a meta-regression study [21] using results from 20 Nordic studies, including our cohort, which could also add to the explanation of why ICH is a male-dominant disease since men have a higher prevalence of hypertension than women in several adult populations [22,23], and men may in general carry a higher atherosclerotic load. However, the findings for childhood BMI in the current study are not in agreement with findings in our previous studies on associations of childhood BMI with hypertension [24] and atherosclerotic diseases, such as ischemic stroke [11] and coronary heart disease [25], where childhood BMI was positively associated with these outcomes in adulthood. Our findings of an association between childhood BMI below average and ICH among men is in accord with a study in adults [26] that found similar associations between low BMI and increased risks of ICH. It is speculated that low cholesterol or other manifestations of cerebral small vessel disease may underlie this association as these are known risk factors of ICH [5]. However, this is speculative and does not explain why it only applies to men.
A potential reason for the sex-difference in the association between birth weight and SAH is smoking, being major risk factor of SAH [5], especially in women [27], and associated with low birth weight. If girls of smoking mothers are more likely to smoke as adults, this could potentially explain why we find an association between lower birth weight and SAH in women in our study.
In general, a plausible mechanism of our findings, although debatable due to the sex differences in the associations, is that poor intrauterine growth may cause permanent structural changes leading to malformations and local weakening of the arterial walls, which increases the likelihood of developing aneurysms. In this context it is interesting to note that the main components of body size, bone, and cartilage are derived from the neural crest, which is also a developmental source of cerebral vasculature. Subsequently, the stress of being very thin and short in childhood, potentially due to a chronic illness and malnutrition, could have negative effects on these possible inborn arterial weaknesses or aneurysms and thus increase the risk of later rupture. However, although these explanations may have played a role, we find them less plausible in the population included in our study, where such health problems are very rare and where children were still well enough to attend school and annual health examinations. Furthermore, the differences in risks between men and women are not in agreement with this general explanation. One of our recent studies, showed that childhood height is inversely associated with ICH in men, but not in women [28], and we found inverse associations of childhood height with SAH in women, but not in men (Supplementary Table 4). Other sex-specific factors and pleiotropic genetic effects on growth and stroke risk deserve further investigations as possible underlying reasons for the associations observed in our study. Moreover, our findings may contribute to the explanation for the higher rates of hemorrhagic stroke in low- and middle-income countries, where stunting in childhood is still a problem.
This is a very large study with prospectively collected data on an unselected population of children from the Copenhagen area. The size and the age structure of the cohort made it possible to examine early life anthropometrics with later risks of rare types of stroke. We used measured as opposed to self-reported BMI, and although birth weight was not measured it was based on reasonably valid recall information [29] from the parents already 7 years after birth. Moreover, the repeated measurements of weight and height allowed us to investigate change in BMI during childhood in relation to ICH. However, due to the low number of cases this analysis could not be performed for SAH. As childhood socioeconomic status is associated with increased risks of stroke and potentially more strongly with hemorrhagic stroke [30], it is possible that socioeconomic status could have influenced our results, but this information was not available in our study. We did, however, not find any differences in the results between birth cohorts despite major changes in living circumstances and distribution of risk factors, such as educational level, income, and marital status that happened during the study period of about five decades. Furthermore, our results remained the same when restricting the sample to those who were diagnosed within the last 10 years, thus eliminating the possibility of questionable diagnoses due to the lack of imaging. The mechanisms underlying the inverse association between birth weight and SAH in women and ICH in men as well as the non-linear association between BMI and ICH among men are unclear, but hypertension, smoking and low cholesterol may be important. It was, however, out of the scope of this study to examine the role of these potential mediating or confounding factors.
Conclusions
Lower birth weight is associated with SAH in women and with ICH in men. Having a BMI below average in childhood or decreasing BMI to low levels during childhood increases the risks of ICH only among men, independently of birth weight. While these findings need to be replicated in populations with different risk factor distributions, they suggest there are sex-specific components in the etiologies of hemorrhagic stroke subtypes.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2018.02033.
Associations of childhood BMI at ages 8, 9, 10, 11, and 12 years with risks of subarachnoid hemorrhage among women and men
Risks of ICH or SAH per 500 g increase in birth weight among women and men diagnosed within the last 10 years of follow-up
Associations of childhood BMI at age 7 and 13 years with risks of SAH among women and men diagnosed within the last 10 years of follow-up
Associations of childhood height at ages 7 to 13 years with risks of SAH among women and men
Associations of childhood body mass index at age 8, 9, 10, 11, and 12 years with risks of intracerebral hemorrhage among women and men. Hazard ratios and 95% confidence intervals (CIs) are given for a 1-unit increase in body mass index (BMI) z-score. All analyses are stratified by birth cohort. (A) Women, 8 years. (B) Men, 8 years. (C) Women, 9 years. (D) Men, 9 years. (E) Women, 10 years. (F) Men, 10 years. (G) Women, 11 years. (H) Men, 11 years. (I) Women, 12 years. (J) Men, 12 years.
Associations of childhood body mass index at age 7 and 13 years with risks of intracerebral hemorrhage among women and men diagnosed within the last 10 years of follow-up. Hazard ratios and 95% confidence intervals (CIs) are given for a 1-unit increase in body mass index (BMI) z-score. All analyses are stratified by birth cohort. (A) Women, 7 years. (B) Men, 7 years. (A) Women, 13 years. (B) Men, 13 years.
Notes
Disclosure
Dr. Line Klingen Gjærde had support from the University of Copenhagen. Dr. Jennifer Lyn Baker was supported by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC (No.281419, childgrowth-2cancer to Dr. Jennifer Lyn Baker) and the European Union’s Horizon 2020 research and innovation programme No.633595, DynaHEALTH. The funders played no role in the preparation of the manuscript or the decision to submit for publication.
Acknowledgements
The Copenhagen School Health Records Register (CSHRR) was established by the former Institute of Preventive Medicine (now the Center for Clinical Research and Prevention). It was built in collaboration with the Copenhagen City Archives in Denmark.