Rate of Stroke Mimics over Telestroke
Article information
Dear Sir:
Intravenous thrombolysis (IVT) remains the cornerstone of acute ischemic stroke treatment [1]. However, use of IVT has remained low due to lacking access to emergency medicine care [2]. Successful implementation of a statewide telestroke network at the Medical University of South Carolina has increased post-IVT transfer (drip-and-ship) from 28 cases in 2008 to 336 in 2015 [3]. A major limitation that consulting neurologists may have during telestroke care is inability to perform first-hand examination before making critical decisions. We investigated whether telestroke is associated with increased frequency of stroke mimics (SM).
A retrospective study was conducted to compare patients who received IVT through telestroke between January 2013 and October 2014 (telestroke group) to those who had IVT directly during the same period (in-house group). Acute ischemic stroke was defined based on either radiological evidence or clinical evidence of symptoms persisting ≥24 hours or until death, and other etiologies excluded. SM was defined as a nonischemic condition mimicking focal ischemic strokes without radiological evidence of acute localized cerebral ischemia relevant to presenting symptoms. To identify SM, we screened cases without acute stroke on 24 hour post-IVT images first. SM cases were identified based on primary discharge diagnoses. Then, a panel with two vascular and two board-certified neurologists reviewed the residual cases to determine them as SM or as neuroimaging-negative cerebral ischemia.
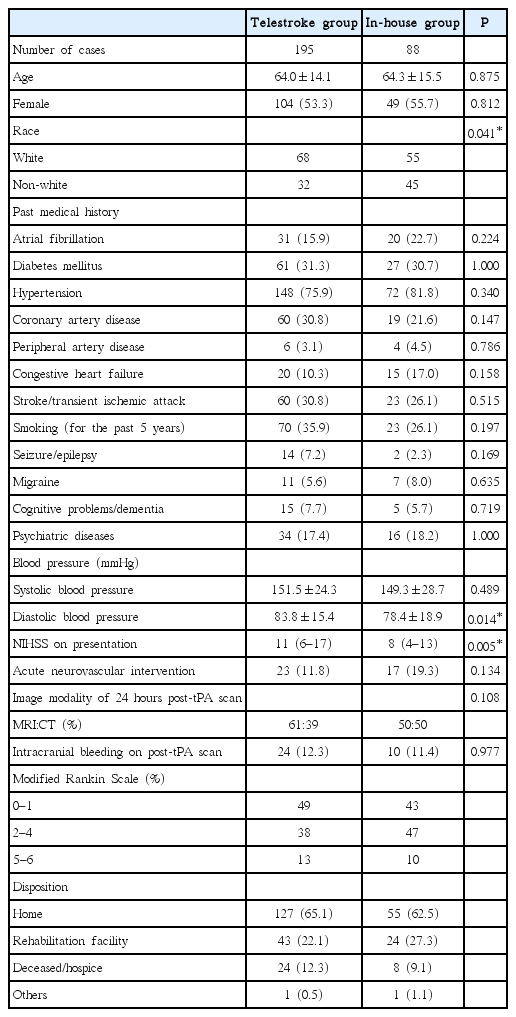
During the study period, 196 patients in the telestroke group and 90 patients in the in-house group received IVT. One case in each group was excluded due to insufficient medical records. Baseline clinical characteristics in each group are shown in Table 1. In the telestroke group, neurological deficit was more severe (median NIHSS, interquartile range: 11, 6–17 vs. 8, 4–13, P=0.005). The rate of post thrombolysis intracerebral hemorrhage was 24 (12.3%) vs. 10 (11.4%) (P=0.977) in the telestroke and in-house groups respectively (Table 1). There was no difference in the percentage of patients achieving good functional outcome (49% in the telestroke vs. 43% in the in-house group).
There were 53 cases (27.2%) and 13 cases (14.8%) of SM in the telestroke and in-house groups respectively (P=0.033) (Table 2). Seventy-eight (40.0%) out of 195 cases in the telestroke group and 30 (34.1%) out of 88 in the in-house did not have radiological evidence of acute stroke on 24 hours post-tPA scans (P=0.415). Logistic regression analysis revealed previous history of seizures, psychiatric conditions and migraine as risk factors of SM (data not shown). SM in both groups combined was associated lower risk of intracranial bleeding (3.0% vs. 18.4%, P<0.0005) and better functional outcomes at discharge (modified Rankin Scale of 0 and 1: 69.7% vs. 40.1%, P<0.0005).
Our study found that telestroke under drip-and-ship paradigm is associated with an increased frequency of SM among patients who undergo IVT, as compared to patients who received IVT after an in-person evaluation.
Telestroke neurologists can encounter the dilemma of “swift or sure” [4] where one may lose chance of good outcome by delaying thrombolysis to gather additional information for diagnostic accuracy. Since it is well known that IVT is relatively safe and functional outcomes are favorable in SM [5], some neurologists may tend to treat a patient with IVT with an unclear clinical scenario. Our telestroke network serves the state of South Carolina, a part of the “stroke belt” of the USA with the highest stroke incidence, and morbidity. This fact may contribute to lower threshold of giving IVT when a diagnosis of acute stroke is unclear but still possible.
The increased frequency of SM could be from the method for case identification. We used a multiple step process to identify SM whereas most studies used one of three methods or in combination including radiological evidence, alternative discharge diagnoses and retrospective review [6]. This difference might have contributed to the higher frequencies of SM in both groups of our study.
Our study also revealed safety and favorable outcome of IVT in SM at the time of discharge. Our study has some limitations, mainly resulting from its retrospective nature. In addition, our study is a single-center study and its result might not be generalizable.
Notes
The authors have no financial conflicts of interest.