Diagnostic and Therapeutic Strategies for Acute Intracranial Atherosclerosis-related Occlusions
Article information
Abstract
Intracranial atherosclerosis-related occlusion (ICAS-O) is frequently encountered at the time of endovascular revascularization treatment (ERT), especially in Asian countries. However, because baseline angiographic findings are similar between ICAS-O and embolism-related occlusion (EMB-O), it is difficult to differentiate the etiologies before the ERT procedure. Moreover, despite successful randomized trials on ERT, results from studies examining the optimal treatment protocol in ICAS-O patients remain unclear. In this review, we describe the clinical and imaging factors that may possibly differentiate ICAS-O from EMB-O. We will also discuss some current hurdles for treating ICAS-O in the hyperacute period and suggest the optimal ERT strategy for ICAS-O patients.
Introduction
The recent success of randomized controlled trials (RCTs) of endovascular revascularization treatment (ERT) for acute ischemic stroke has led us to consider ERT as a standard of care in clinical practice [1-6]. The main reasons for the success of RCTs have been 1) the development of effective and safe thrombectomy devices, 2) the appropriate selection of patients based on brain and vessel imaging, including the ischemic core/penumbra, and the status of collateral circulation, and 3) the shortened door-to-procedure time [7]. It is noteworthy that the newer thrombectomy devices, such as a stent retriever or direct aspiration system, have enabled us to achieve an improved reperfusion rate, which is strongly associated with better clinical outcomes [8]. However, these thrombectomy devises are primarily designed for recanalization of the occluded artery by removing an embolus [9-11], and recanalization may not be sufficiently achieved if a significant stenosis, related to intracranial atherosclerosis (ICAS), is present at the occlusion site [12].
While a recent French study revealed that patients with underlying ICAS accounted for 5.5% of patients who underwent ERT [13], underlying ICAS appears to be more frequent in Asian countries; a Korean study reported that around 15% of ICAS-related occlusions (ICAS-O) are found in patients with intracranial artery occlusion in anterior circulation [14]. Another Korean study demonstrated that ICAS-O was encountered in approximately 35% patients with vertebrobasilar artery occlusion [6]. Thus, the ICAS-O should not be ignored in Asian countries.
One of the biggest issues surrounding ICAS is the difficulty in differentiating ICAS-O from embolism-related occlusion (EMB-O) through baseline angiography; the presence of ICAS-O is usually identified only during the ERT procedure. Nevertheless, recent studies have identified some factors that may differentiate the two conditions. In this review, we describe the characteristics of ICAS-O, as well as the clinical and imaging findings that may help us to predict ICAS-O. We will also suggest an appropriate ERT strategy for ICAS-O based on literature review and our experiences.
Predictors of ICAS-O
Clinical predictors
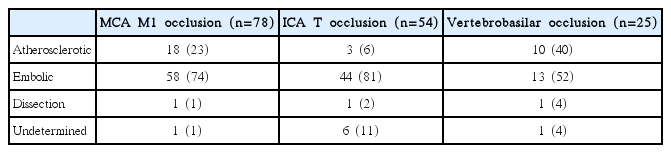
In a retrospective single center study, there were more males in the ICAS-O than in the EMB-O patients, as determined by univariate analysis (91.7% vs. 53.7%, P<0.001) [14]. In another study, which included middle cerebral artery (MCA) M1 occlusions, ICAS-O were more frequent in male patients, although the statistical significance was marginal (67.5% vs. 51.2%, P=0.072) [15]. In stroke patients with MCA M1 occlusions, the baseline NIHSS score was significantly lower in ICAS-O patients than in EMB-O patients (median, 14.5 vs. 16.0, P=0.006) [15]. In the posterior circulation, the initial median NIHSS score also tended to be low in the ICAS-O patients than in the EMB-O patients (14 vs. 22, P=0.096) [6]. In terms of circulation territory, ICAS-O was relatively more common in posterior circulation than in anterior circulation [14]. Specifically, the ERT database in Ajou University Hospital (Suwon, Korea, January 2011 to February 2016] showed that ICAS was most frequently observed in vertebrobasilar artery system (40%), followed by MCA M1 (23%). ICAS was infrequent in internal carotid artery (ICA) T occlusions (6%; unpublished data; Table 1).
Risk factors differ between ICAS-O and EMB-O. In one study, high total cholesterol levels were independently associated with ICAS-O (odds ratio 1.019 per 1 mg/dL of total cholesterol level, 95% confidence interval 1.005–1.033, P=0.008) [14]. Smoking was also more closely associated with ICAS-O than EMB-O (50.0% vs. 13.4%, P<0.001) [14]. In a study on MCA M1 occlusions, current smoking was more frequent in the patients with ICAS-O than in those without (57.5% vs. 26.8%, P<0.001) [15]. The frequency of hypertension or diabetes mellitus did not differ between groups in either study [14,15]. Atrial fibrillation is strongly related to EMB-O (0% vs. 59.7%, P<0.001 and 0% vs. 63.4%, P<0.001 in References 14 and 15, respectively) [14,15]. However, the absence of atrial fibrillation does not necessarily indicate an ICAS-O; cardioembolism may sometimes co-exist with underlying ICAS. Interestingly, cryptogenic embolism accounts for a substantial portion of EMB-O patients (18.7%). Artery-to-artery embolism accounts for a small portion of EMB-O patients (3.7%) [14].
Imaging predictors
Clot signs and clot burden
The gradient echo (GRE) susceptibility vessel sign has been shown to be associated with cardioembolic stroke [16]. However, a more recent study showed that the frequency of GRE susceptibility vessel sign does not differ between ICAS-O and EMB-O (71.4% vs. 84.1%, P=0.239) [17]. This result was consistent with a previous study that reported a high frequency of GRE susceptibility vessel sign (60.5%) in patients with ICAS-O, due to in situ thrombosis [18]. The only difference identified was the clot burden score, which was smaller in ICAS-O than in EMB-O (median clot burden score on GRE [higher scores indicate smaller burden], 8 vs. 6, P=0.009) [17]. These findings suggest that ICAS-O may be suspected when the clot burden is small.
DWI infarct volume and patterns
Baseline infarct core volume is reported to differ between ICASO and EMB-O; in one study, the median infarct core volume was 14 mL and 54 mL in ICAS-O and EMB-O, respectively (P<0.001) [17]. This may be due to pre-existing collateral circulation in patients with ICAS-O. At Ajou University Hospital (Suwon, Korea), we attempted to evaluate leptomeningeal collaterals identified through digital subtraction angiography prior to ERT (according to American Society of Interventional and Therapeutic Neuroradiology collateral grading) in patients with MCA M1 occlusions. The best collateral grade (full and rapid) was found in 8 of 10 patients (80.0%) in ICAS-O patients, which was significantly higher (P=0.041) than that of EMB-O patients (19 of 43 patients; 44.2%; unpublished data). Additionally, comparisons of diffusion-weighted imaging (DWI) of the baseline infarct pattern between these groups shows that EMB-O patients more frequently display a large territorial infarct pattern than ICAS-O patients (55.1% vs. 18.2%, P=0.020), whereas border-zone (12.2% vs. 36.4%, P=0.032) and scattered (30.6% vs. 54.5%, P=0.110) infarct patterns were less frequent in EMB-O than in ICAS-O patients [17]. Thus, identifying infarct patterns on DWI may help us to differentiate between these two conditions. Interestingly, two distinctive types of baseline DWI lesions are often seen in ICAS-O patients. These are scattered and border-zone infarctions, with brighter signal, and perforator infarctions, with less bright signal. A representative case is illustrated in Figure 1.
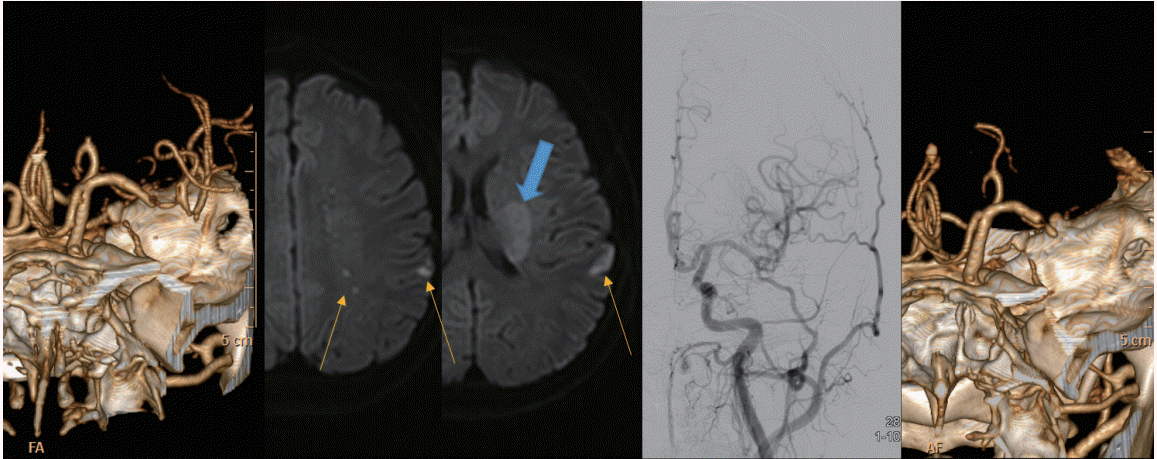
A representative case of ICAS-O with characteristic infarct patterns. A 27-year-old male patient showing bright scattered and external border-zone infarctions (yellow arrows) and less bright perforator infarctions (blue arrow). After several thrombectomy trials, successful reperfusion was achieved, and the patient’s neurological deficits were mostly resolved. Although the vessel was re-occluded on the repeat angiography the next day, the patient’s neurological status was not changed. This phenomenon suggests that delayed occlusion of perforators, due to the propagation of in situ thrombosis, can cause more direct neurological deficits. ICAS-O, intracranial atherosclerosis-related occlusion.
Vessel calcification
The frequency of arterial calcification, observed through noncontrast computed tomography (CT) from the data in Ajou University Hospital (Suwon, Korea; from January 2010 to March 2014), was not different between the ICAS-O and EMB-O patients in the anterior circulation (0% vs. 14%, P=0.145; unpublished). However, calcification along the vertebrobasilar artery was more often observed in ICAS-O than EMB-O in patients with posterior circulation stroke (none, 16.7% vs. 58.1%; calcification in situ, 27.8% vs. 12.9%; calcification proximal to the occlusion, 55.6% vs. 29.0%; P=0.018) [6].
Other findings obtained during the procedure
Information obtained during the ERT procedure may give us some clues towards the underlying cause of occlusion. First, angiogram findings may reveal the full and rapid leptomeningeal collaterals in patients with ICAS-O as described above (see “DWI infarct volume and patterns”). Second, in the case of a stent deployed prior to retrieval, the shape in the target occlusion site could be divided into truncal and branching types [19]. A truncal-type occlusion site was associated with more frequent stent retrieval failure and a negative embolic source, even after intensive work-ups [7], suggesting that this type of occlusion is associated with ICAS-O.
Suggestions for the identification of ICAS-O
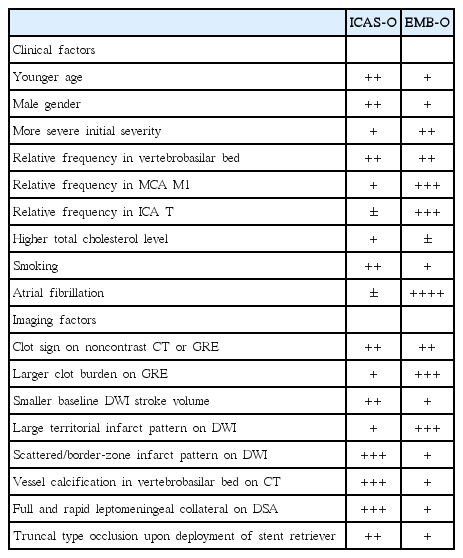
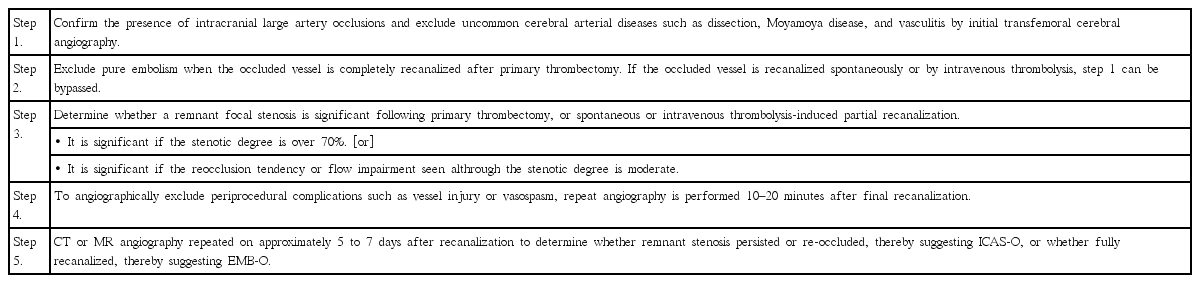
With clinical and imaging factors summarized in Table 2, we can roughly predict ICAS-O before ERT procedures. ICAS-O can be further diagnosed on the basis of the emergent digital subtraction angiography (DSA) and images taken during the ERT procedure (Table 3) [6]. With DSA, rare diseases such as dissection, moyamoya disease and vasculitis should be excluded. EMB-O and ICAS-O can then be differentiated by a few passes of a mechanical thrombectomy device. Stent retrievers may be more advantageous for detecting underlying ICAS than direct clot aspiration devices, given the results of a study demonstrating that truncal-type occlusions were more closely associated with ICAS (as described under “Other findings obtained during procedure”) [19]. If a complete recanalization of the target vessel is achieved, the case can be defined as an embolic occlusion. Whereas, the presence of a residual stenosis suggests the presence of ICAS-O. ICAS-O has a strong tendency to reocclude, so repeat angiography 10–20 minutes following the thrombectomy would be reasonable. Repeat CT or MR angiography several days after ERT may show either remaining stenosis or reocclusion in ICAS-O patients, or more complete recanalization of mild remnant thrombotic stenosis in EMB-O patients, thus helping us to identify the underlying etiologies.
Therapeutic strategy for acute ICAS-O
It has been reported that an ICAS-O is an independent predictor for poor outcomes in patients with acute vertebrobasilar occlusions and ERT [6]. This link may be attributed to the longer procedure duration and additional rescue treatment required in ICAS-O, and the neighboring perforators occlusion during ERT. In the anterior circulation, single center retrospective studies have shown that ICAS-O was associated with similar or slightly improved outcomes than EMB-O, through univariate analysis. However, this finding may be attributed to the favorable baseline characteristics, such as younger age and less severe neurologic deficits, in the ICAS-O patients [14,15].
The authors would like to suggest some ERT strategy of ICAS-O, considering it is important to reduce the time for additional rescue treatments and to prevent perforator occlusions during procedure (Figure 2). First, the removal of in situ thrombi by thrombectomy is important in ICAS-O (Figure 1). In situ thrombi can be propagated in a no-flow vessel and may further occlude perforating vessels resulting in severe neurologic deficits (Figure 2A-C). Therefore, a first-line thrombectomy using routine devices would be reasonable (Figure 2D). There are concerns regarding endothelial damage due to friction from thrombectomy devices on the atherosclerotic vascular surface [20-22]. However, stent retrievers have been generally reported to be safe, and appear to be feasible for the treatment of ICAS-O [12].
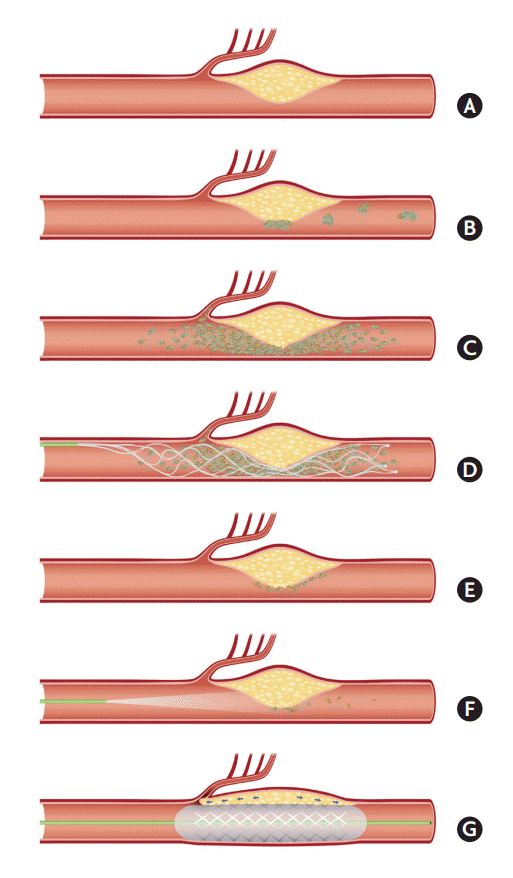
Illustrations of ICAS-O in terms of stroke pathomechanism and ERT strategy. (A-C) Pathomechanism of cerebral infarction on ICAS-O. Border-zone and scattered infarctions can occur from some microemboli, from in situ thrombosis in ICAS lesion. Perforator infarctions can also occur from the propagation of the thrombosis. (D) Stent retrieval for ICAS-O. Routine first-line thrombectomy can effectively eliminate the major portion of in situ thrombi. (E and F) Endothelial cells are still inflamed and may cause reocclusion. Glycoprotein IIb/IIIa inhibitor can stabilize the irritable endothelium. (G) The location of nearby important perforators should be cautiously evaluated when angioplasty and/or stenting are considered. This procedure can block the perforators, thereby aggravating neurological deficits. ICAS-O, intracranial atherosclerosis-related occlusion; ERT, endovascular revascularization treatment.
Second, stabilization of ICAS endothelium seems to be important. Inflammatory responses occur in the stenotic or ruptured atherosclerotic plaque lesions, which have been well described in vulnerable coronary and carotid plaques [23,24]. This irritable endothelial status may cause re-occlusion of the recanalized vessels (Figure 2E) [12,25,26]. Procoagulant activity may increase in stroke patients who are given intravenous tissue plasminogen activator [27]. Therefore, antiplatelet treatment may be more useful in patients with irritable and vulnerable vascular lesions than fibrinolytics, such as tissue plasminogen activator or urokinase [25]. Local infusion of an antiplatelet agent, tirofiban, to the in situ thrombotic occlusive lesion was shown to be safe, to reduce re-occlusion events, and to improve reperfusion rates (Figure 2F) [26]. Glycoprotein IIb/IIIa inhibition blocks fibrinogen molecules, which form bridges between adjacent platelets, providing an effective antiplatelet function [28]. Abciximab, the glycoprotein IIb/IIIa inhibitor, has an irreversible action on platelets, whilst other inhibitors, such as tirofiban and eptifibatide, have a reversible action and may reduce periprocedural hemorrhagic complications [28]. A recent open-label non-randomized study found that hemorrhagic complications did not increase in acute stroke patients who undertook 3 days of intravenous tirofiban infusion immediately following intravenous tissue plasminogen activator, compared to patients who were given intravenous tissue plasminogen activator only [29]. The use of glycoprotein IIb/IIIa inhibitors appears to be potential safe and effective treatment, although further randomized clinical trials are needed to confirm their efficacy.
Third, angioplasty using balloons and/or stenting should be selectively considered as the next line of treatment. However, it is possible that branch perforators can be occluded during angioplasty/stenting via a so-called “snowplow” effect, similar to a snowplow truck that itself compresses snow and blocks a sinkhole while removing snow. In fact, a randomized controlled ICAS study (SAMMPRIS) found that percutaneous stenting/angioplasty used in combination with the best medical treatment shows poorer outcomes than when the best medical treatment alone [30]. In this study, among the 19 patients with periprocedural ischemic complications, 12 patients had perforator occlusions and 2 patients had mixed perforator and embolic occlusions [31]. In addition, the interventional treatment for occlusions in the basilar artery had an odds ratio of 6.2 for periprocedural ischemic events when compared to occlusions of other vessels [31]. Despite the concerns of branch occlusion, emergent angioplasty, with or without stenting in the hyperacute period of intracranial large artery occlusion, has been shown to be feasible and have favorable outcomes [32]. In intractable intracranial occlusions treated with detachable stent retrievers, permanent stenting by detaching the just-used stent retriever improves reperfusion rates and clinical outcomes [33]. To achieve these better outcomes, clinicians should determine whether important branches are located near the ICAS lesion (Figure 2G). If important branches are located near the remnant ICAS site and are predicted to be prone to occlusion during angioplasty/stenting, it would be better not to perform the treatment. In addition, clinicians should keep in mind that antiplatelet pretreatment prior to stenting is related to less recoil and in situ thrombotic complications, as well as better outcomes in early percutaneous coronary intervention treatments for coronary artery obstructive disease [34]. In reality, however, antiplatelet pretreatment is difficult to administer to patients preparing for an emergent ERT. In addition, hemorrhagic transformations from large cerebral infarctions can inhibit further antiplatelet treatment. In this situation, in situ thrombosis is especially likely in patients with intracranial stenting in the hyperacute period. Therefore, intracranial stenting as a rescue method during the hyperacute period should be considered last and only in selected patients with relatively small infarct volumes, which are less associated with hemorrhagic transformation, and those who have not been treated with intravenous fibrinolytic agents prior to stenting. These conditions allow for continued antiplatelet administration, which is required to maintain intracranial stenting. Cerebral infarcts due to hemodynamic impairments are better candidates for intracranial stenting than those due to branch atheromatous diseases and thromboembolic occlusions, which may underlie ICAS [35].
Postprocedural medical treatment management by attending physicians is also of substantial importance. The current best medical treatments, which are based on strong antiplatelet and intensive statin administration for ICAS, appear to lead to stenosis regression more often than expected, even without intervention. Around 80% of patients with ICAS have regression of the stenosis within 1 year of occlusion [36]. Therefore, dual antiplatelet and intensive statin treatment are necessary to prevent remnant ICAS stenosis after finishing ERT. However, the above treatments should be applied cautiously during the immediate postprocedural period, especially when ERT follows intravenous fibrinolytic treatment. Intravenous maintenance with a reversible antiplatelet agent, such as tirofiban, may be a good treatment option, although more evidence regarding its safety and efficacy is required.
Future perspectives
Toward better imaging
ICAS-O is not a rare disease entity in Asian countries. First, clear differentiation between ICAS-O and EMB-O is urgently required. Currently, vessel wall MRI and direct thrombus imaging are candidates. Studies investigating these methods are ongoing.
Vessel wall MRI
High resolution MRI, which allows for the vessel wall to be seen in detail, can be a good option for identifying underlying cause of arterial occlusion [37-39]. On cross-section images, an eccentric atherosclerotic plaque, with or without contrast enhancement, can be seen in ICAS. This is sometimes seen along-side intraplaque hemorrhage [37]. In a postmortem study, a low signal-intensity area on T1-weighted fat-suppressed imaging was found to represent the lipid core of intracranial atherosclerosis [40]. Although these findings mostly come from studies of stenotic ICAS lesions rather than ICAS-O, it is thought that the findings are similar between ICAS and ICAS-O. However, because in situ thrombosis may contaminate the MRI findings, further studies are needed to elucidate findings that distinguish ICAS-O from EMB-O. In embolic occlusion, conversely, vessel wall MRI has not been studied well. An example of embolic infarction in the vertebral artery territory due to atrial fibrillation (Figure 3), shows that eccentric stenosis or plaque is not seen on the vessel wall of the occluded artery. A different signal intensity on T1-weighted with or without contrast and proton-density imaging is seen in the occluded arterial lumen compared to the contralateral non-occluded vessel, suggesting the presence of an embolus. Nevertheless, physicians should try to reduce image to puncture time in the ERT field. This high-technology imaging needs much more time and more contrast before ERT is started, thus, a selective specific imaging protocol may have to be developed for potential use of these MRI findings in differentiating between the two conditions [41].

An embolism-related occlusion (EMB-O) case with vessel wall MRI taken on the third day after stroke onset. A 72-year-old man had an acute infarction in the posterior inferior cerebellar artery territory, due to right vertebral artery occlusion, but did not undertake endovascular revascularization treatment (ERT). His stroke etiology was cardioembolism by thorough evaluations. (A) Contrast-enhanced MR angiography shows an occlusion in the right vertebral artery. (B) Gradient echo images taken on admission, included in the baseline routine MRI, show a susceptibility vessel sign. Proton density-weighted (C) and T1-weighted (D) imaging does not show a specific finding but an occlusion is suggested since a signal void, as seen in normal arterial lumen, is absent. (E) T2-weighted imaging reveals an intact vessel wall but the lumen shows slightly higher signal intensity compared to a normal contralateral vertebral artery. (F) Thrombus in the occluded vessel appears to be stained by contrast and shows high signal intensity. The blue arrow indicates the vertebral artery occluded due to an embolism. The yellow arrow indicates the contralateral normal vertebral artery.
Direct thrombus imaging
Direct thrombus imaging is currently being developed in the experimental setting. This work may enable us to differentiate between ICAS-O and EMB-O in the future [42]. There are some candidates that can be used as a contrast material for fibrin in a thrombus. Using fibrin-targeted glycol-chitosan-coated gold nanoparticles, the thrombus can be directly delineated on serial CT imaging [43]. Using contrast with EP02104R, the thrombus can be seen at intracranial arterial occlusion sites in rats [44]. These materials are sensitive to fibrin; therefore, the results may differ based on the type of thrombus that is present. However, thrombus imaging is not approved for clinical practice at the time of writing. As a result, further studies are required to enable the application of such imaging techniques in our clinical practice.
Toward better practice
The ERT method, used to treat ICAS-O, should undergo more studies in order to improve its outcomes. Further studies regarding ERT treatment and prognostic improvement may be helped by the use of a consistent definition system. First, approved mechanical thrombectomy techniques, such as stent retrieval and direct clot aspiration require further verification in patients with ICAS-O. Second, the optimal rescue treatment for the remnant atherosclerotic stenosis should be determined from among local antiplatelet infusion, balloon angioplasty, and permanent stent deployment. Third, detachable stent retrievers for permanent deployment should be evaluated further to ensure that their radial forces are sufficient to endure the remaining ICAS stenosis. In summary, we predict that the practical issues regarding the treatment of ICAS-O can be solved within the next couple of years, and that the prognosis can be improved to match that of a typical embolic cases.
Notes
The authors have no financial conflicts of interest.