Spontaneous Intracerebral Hemorrhage: Management
Article information
Abstract
Spontaneous non-traumatic intracerebral hemorrhage (ICH) remains a significant cause of mortality and morbidity throughout the world. To improve the devastating course of ICH, various clinical trials for medical and surgical interventions have been conducted in the last 10 years. Recent large-scale clinical trials have reported that early intensive blood pressure reduction can be a safe and feasible strategy for ICH, and have suggested a safe target range for systolic blood pressure. While new medical therapies associated with warfarin and non-vitamin K antagonist oral anticoagulants have been developed to treat ICH, recent trials have not been able to demonstrate the overall beneficial effects of surgical intervention on mortality and functional outcomes. However, some patients with ICH may benefit from surgical management in specific clinical contexts and/or at specific times. Furthermore, clinical trials for minimally invasive surgical evacuation methods are ongoing and may provide positive evidence. Upon understanding the current guidelines for the management of ICH, clinicians can administer appropriate treatment and attempt to improve the clinical outcome of ICH. The purpose of this review is to help in the decision-making of the medical and surgical management of ICH.
Introduction
Spontaneous non-traumatic intracerebral hemorrhage (ICH) is the second most prevalent subtype of stroke and is associated with high mortality and morbidity throughout the world [1-3]. Various clinical trials related to the medical and surgical management of ICH have been conducted to overcome its devastating clinical course. Despite these efforts in the past decades, there have been no dramatic advances in the development of interventions to improve the functional outcomes after ICH [4]. In this situation, many clinicians may misunderstand that effective treatment options are lacking; however, the necessity of excellence in clinical care and research should be emphasized rather than underestimated. In this review, we discuss previous clinical trials and the current guidelines for the management of spontaneous ICH; ongoing clinical trials are also included. For this purpose, a systematic literature review was conducted with full PubMed searches for all the English articles about the management of ICH, regardless of the date of publication.
Initial evaluation and management
In terms of the pathogenesis of ICH resulting from bursting of intracerebral arteries, a majority of fatalities occur in the first two days of the onset of symptoms [5,6]. Furthermore, nearly one-fifth of the patients with ICH experience neurological deterioration in the pre-hospitalization period [7], and one-fourth of the patients in the hospitalization period [8]. Rapid initial diagnosis and concentrated management are crucial in the early management of ICH. When a patient presents with focal neurological deficits, severe headache, vomiting, high systolic blood pressure (SBP) greater than 220 mmHg, and decreased consciousness with a sudden onset, ICH should be the first condition considered in the diagnosis. In addition to clinical presentation, a brief medical history including hypertension, prior stroke, recent head trauma, and prior use of antithrombotic drugs including anticoagulants, should also be recorded. After a quick assessment of the medical history and presentation, neuroimaging should be performed to confirm the diagnosis. Brain computed tomography (CT) is the gold standard for identifying acute hemorrhage; magnetic resonance imaging (MRI) can be an alternative with an advantage of being able to differentiate between the acute and chronic stages of hemorrhage [9,10].
In addition to the initial diagnosis of ICH at the emergency room, acute management should be cooperated at the same time. The main principle for the early management of ICH is the same as that for the management of ischemic stroke [11]. Airway management (if needed), cardiovascular support, urgent BP lowering treatment, and reversal of coagulation abnormalities should be initiated at the emergency room. Critical protocols developed for the management of ICH may allow more efficient, standardized, and integrated management of patients with ICH and reduce the length of stay at the emergency room by facilitating their prompt admission to a stroke unit or a neuroscience intensive care unit [12].
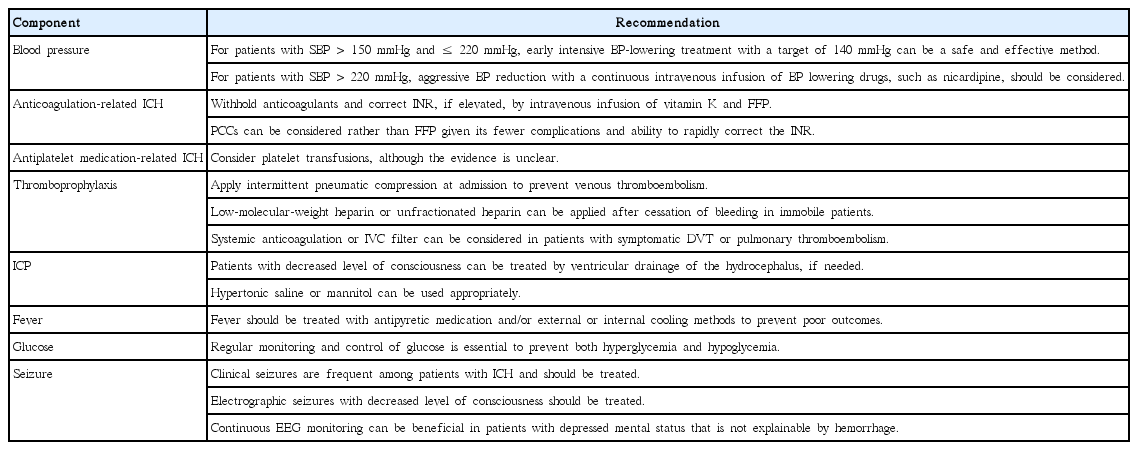
Various grading scales are used for predicting the prognosis in the early stages of ICH [5,13-17]. The ICH score is the mostly commonly used scale and can be easily calculated based on neurological examination and CT findings (Table 1) [5]. In prospective observational cohort studies, the ICH score could be a valid clinical grading scale for the 30-day mortality (Figure 1) [5] and 12-month functional outcome [15].
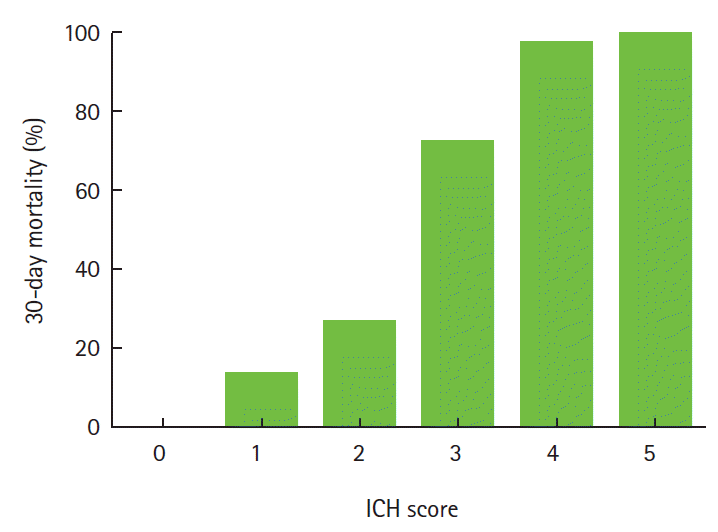
The ICH Score and 30-day mortality. Data were revised from Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891-897. There was no patient with a score of 6 in the cohort, but an ICH score of 6 would be predicted to be associated with a high risk of mortality.
Medical management
Recommendations for medical management of ICH are summarized in Table 2 and described below.
Monitoring and nursing care
The condition of patients with ICH frequently deteriorates within the first 24 or 48 hours after symptom onset because of secondary injuries caused by hematoma expansion, intraventricular hemorrhage (IVH) extension, fever, and high blood pressure [18-20]. Hence, patients in the acute phase of ICH should be monitored and taken care of in facilities in which the close monitoring of the patient’s status and frequent administration of medications are possible. In a prospective observational study, the patients admitted to a specialized neuroscience intensive care unit (ICU) showed reduced mortality compared to those admitted to the general ICU [21]. In a Swedish cohort study with 86 hospitals and 105,043 patients, care in the stroke unit was associated with better long-term survival in patients with ICH [22]. Specialized care units such as the neuroscience ICU and stroke unit can provide close monitoring of blood pressure (BP), heart rate, electrocardiograph findings, oxygen saturation, and neurological status in medically and neurologically unstable patients in the early stage of ICH. The intracranial pressure (ICP), cerebral perfusion pressure, and continuous intra-arterial blood pressure (BP) can also be monitored.
Blood pressure reduction
Based on the viewpoint that increased BP causes greater tearing of blood vessels and flow-out of blood through these vessels and eventually leads to the expansion of the hematoma, high BP is considered to be associated with hematoma expansion and poor outcomes, especially early neurological deterioration, mortality, and dependency [23-25]. Thus, intensive BP reduction is thought to reduce hematoma expansion and improve the clinical outcomes in patients with ICH. However, the therapeutic goals of BP reduction in the early phase of ICH are not clearly defined. The key point to debate is whether acute BP reduction results in ischemic insult to perihematomal penumbral lesions surrounding the hemorrhage [26]. On the other hand, a randomized clinical trial showed that rapid BP reduction targeting an SBP of <150 mmHg did not reduce perihematomal cerebral blood flow on CT perfusion imaging further than that targeting an SBP of <180 mmHg [27].
Recently, a few randomized clinical trials were performed to identify therapeutic targets and evaluate the safety of intensive BP reduction in the early phases of ICH [28-30]. The pilot phase of the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage (INTERACT 1) trial enrolled 404 patients with acute spontaneous ICH within 6 hours of symptom onset from Australia, China, and South Korea [29]. They compared the early intensive BP lowering group (target SBP, 140 mmHg) to the standard guideline-based group (target SBP, 180 mmHg). The primary outcome was proportional change in the hematoma volume at 24 hours. In 2008, the results showed that the mean hematoma expansion at 24 hours was greater (P=0.04) in the guideline-based group (36.3%) than in the intensive BP lowering group (13.7%) [29,31]. After controlling for the effects of the initial hematoma volume and the onset-to-CT time, the median hematoma expansion was 16.2% in the guideline-based group and 6.2% in the intensive BP lowering group (P=0.06) [29]. There were no differences in the 90-day functional outcomes and adverse events between the two treatment groups [29].
In 2010, the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trial reported similar results about the safety of early intensive BP lowering treatment. This study was an open-label pilot study designed to evaluate the feasibility and safety of three escalating levels of antihypertensive treatment with the intravenous administration of nicardipine in patients with ICH-related acute hypertension [30]. Patients with ICH and an SBP ≥170 mmHg who presented within 6 hours of the onset of symptoms were enrolled. Continuous intravenous nicardipine was administered to achieve a target SBP of 170–200 mmHg in the first group (n=18), 140–170 mmHg in the second group (n=20), and 110–140 mmHg in the third group (n=22). The investigators found no significant relationship between BP reduction and any of the outcome measurements (hematoma expansion, higher perihematomal edema ratio, and poor 3-month modified Rankin scale score).
Recently, the results of the largest randomized clinical trial evaluating the efficacy of intensive BP lowering were published [28]. The INTERACT 2 trial is a phase 3 trial enrolling 2,839 patients with an SBP between 150 and 220 mmHg within 6 hours of the ICH. The participants were randomized to the intensive treatment group (target SBP, <140 mmHg) or the standard treatment group (target SBP, <180 mmHg). The BP lowering therapy was started within 1 hour of randomization and continued for a duration of 7 days. The investigators found that the intensive treatment group was less likely to have the primary outcome of death or major disability (modified Rankin scale score ≥3; OR [odds ratio], 0.87; 95% CI [confidence interval], 0.75–1.01; P=0.06) [4,28]. Ordinal analysis showed that the intensive treatment decreased the odds of higher modified Rankin scale scores (OR, 0.87; 95% CI, 0.77–1.00; P=0.04) [28]. The incidence of nonfatal serious adverse events did not differ between the two treatment groups.
In 2016, the results of the ATACH 2 trial [32] were reported. The purpose of this trial was to determine the efficacy of rapidly lowering SBP in patients with ICH in an earlier time window than that evaluated in previous trials. A total of 1,000 patients with ICH were randomized to intensive BP lowering (target SBP, 110-139 mmHg) or standard BP lowering (target SBP, 140-179 mmHg). Intravenous nicardipine within 4.5 hours of the onset of symptoms was used as a method of BP reduction, which was earlier compared to the 6-hour time-point in the INTERACT 2 trial. The primary outcome of death or disability (modified Rankin scale score of 4 to 6) at 3 months after randomization was achieved in 38.7% of the patients in the intensive treatment group and in 37.7% of the patients in the standard treatment group (adjusted relative risk [RR], 1.04; 95% CI, 0.85-1.27; P=0.72) [32]. While the incidence of hematoma expansion, defined as a >33% increase in the ICH volume over the initial 24 hours after the onset of symptoms, was lower in the intensive treatment (18.9%) group than in the standard treatment group (24.4%), the difference between the groups was not statistically significant (P=0.08). There was no significant difference in the incidence of treatment-related serious adverse events within 72 hours of the onset of symptoms.
Overall, the current evidence supports that early intensive BP lowering is safe and feasible, and is associated with a modestly better functional outcome. The 2015 American Heart Association/American Stroke Association guidelines for the management of spontaneous ICH recommend early BP reduction with an SBP target of 140 mmHg for patients with ICH presenting with an SBP between 150 and 220 mmHg and without any contraindication to acute BP treatment [4]. For patients with ICH presenting with an SBP >220 mmHg, aggressive BP reduction with continuous intravenous infusion and frequent BP monitoring may be reasonable.
Anticoagulation-related intracerebral hemorrhage
The clinical outcomes of vitamin K antagonists (VKA)-related ICH are poor. Therefore, in addition to stopping VKA, urgent measures are usually needed to reverse the effects of VKA in patients with an elevated international normalized ratio (INR) [2]. Intravenous vitamin K administration at a dose of 5-10 mg should be initiated in the first hours of symptom presentation. Fresh-frozen plasma, along with vitamin K, has been used for the rapid correction of INR for years. Recently, prothrombin complex concentrates (PCCs), activated PCC factor VIII inhibitor bypassing activity (FEIBA), and recombinant activated factor VIIa (rFVIIa) have been evaluated as potentially more effective alternatives [4].
Reversal of non-vitamin K antagonist oral anticoagulants (NOACs)-related ICH has been poorly evaluated. Recently, idarucizumab, a monoclonal antibody designed for the reversal of anticoagulant effects of dabigatran, has been introduced into medical practice as the first antidote for NOACs [33,34]. Furthermore, hemodialysis can be used in case of dabigatran [35]. Andexanet alfa, a specific reversal agent to neutralize the anticoagulant effects of factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban, is presently in phase III clinical trials [35-37]. Potential reversal strategies using FEIBA, other PCCs, rFVIIa, or activated charcoal might be considered [4,35].
In contrast to VKA, NOACs do not require hematological monitoring because they have different effects and sensitivities on screening coagulation tests including the prothrombin time (PT), activated partial thromboplastin time (aPTT), and thrombin clotting time (TT) [38,39]. Residual effects of NOACs can be estimated based on the elimination half-life of each NOAC and the renal function of individuals on NOACs [39]. When the NOAC status of the patient is unknown, a coagulation assay can be performed to estimate the presence and concentration of NOACs in the body. Dabigatran is a direct thrombin inhibitor, and a high aPTT at trough may be associated with a higher risk of bleeding, and a normal aPTT in dabigatran-treated patients has been used in emergency situations to exclude any residual anticoagulant effect [39-42]. Rivaroxaban, a direct factor Xa inhibitor, can prolong the PT significantly [43]. Apixaban, another direct factor Xa inhibitor, may be associated with prolonged PT and aPTT, but normal PT and aPTT do not rule out significant anticoagulant effects [39]. Thus, a drug-specific anti-factor Xa chromogenic assay is necessary [44].
As the optimal timing for resumption of anticoagulation after anticoagulation-related ICH is unknown, the risk of both cardioembolic events and recurrent anticoagulation-related ICH should be considered together in determining the starting time of anticoagulation [45,46]. In general, resumption of VKA within the first month is associated with a high risk of recurrent ICH [47]. Therefore, a delay of at least 1 month should be suggested in patients with VKA-related ICH [4]. However, early resumption of anticoagulation may be needed in patients with prosthetic heart valves because of the high risk of cardioembolic events [4]. In patients with lobar ICH, resumption of anticoagulation is reported to be associated with higher risk of recurrent ICH compared with deep hemispheric hemorrhage (1-year risk of recurrence, 15% versus 2.1%) [48,49]. Therefore, avoidance of long-term anticoagulation with VKA as a treatment for nonvalvular atrial fibrillation is probably recommended in patients with VKA-associated lobar ICH [4]. The safety of antiplatelet agents as alternatives to VKA in patients with lobar ICH is controversial [50,51]. In patients with non-lobar ICH, antiplatelet monotherapy can be a safer alternative to VKA in some patients with atrial fibrillation [4,51,52]. As alternatives to VKA after ICH, the usefulness of NOACs including dabigatran, rivaroxaban, and apixaban, remains unknown [4,53-55].
Antiplatelet medication-related intracerebral hemorrhage
The effect of antiplatelet drugs on the outcome of ICH is uncertain. Two observational studies showed that reduced platelet activity is associated with IVH, death, early ICH growth, and poor functional outcome [56,57]. Another study reported that the use of antiplatelet medication at the onset of ICH symptoms was not associated with increased hemorrhage volume, hematoma expansion, or poor functional outcome [58]. Platelet transfusion might be considered in patients with acute ICH with prior antiplatelet use or platelet dysfunction, although there has been no randomized controlled trial testing this. Two trials on platelet transfusion in patients with ICH are ongoing [2,59].
Thromboprophylaxis in intracerebral hemorrhage
Thromboprophylaxis in patients with acute ICH is a complicated problem. The risk of bleeding is not low, and the risk of venous thromboembolism is high. Therefore, thromboprophylaxis should not be delayed [4]. The incidence of symptomatic venous thromboembolism ranges from 0.5% to 13%, while that of pulmonary embolism ranges from 0.7% to 5% [60]. Methods for thromboprophylaxis were suggested through 3 different trials termed the CLOTS trials (Clots in Legs or Stockings After Stroke) I-III [61-64]. The results suggested that intermittent pneumatic compression started as early as the day of hospitalization could reduce the occurrence of proximal deep vein thrombosis (DVT), and that graduated compression stockings were not effective.
Systemic anticoagulation or inferior vena cava (IVC) filter placement are probably indicated in patients with ICH with symptomatic DVT or pulmonary embolism [4]. One meta-analysis found that early anticoagulation, including low-molecular-weight heparin or unfractionated heparin or heparinoids, is associated with a significant reduction in pulmonary embolism and a non-significant increase in hematoma enlargement [65]. An IVC filter is usually not recommended in patients with acute DVT of the leg in addition to anticoagulant therapy. However, if anticoagulation therapy is contraindicated, an IVC filter can be used [66].
ICP and cerebral edema management
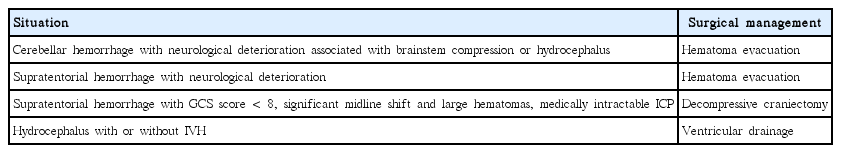
Intracranial hypertension following ICH is common, especially in younger patients with supratentorial hemorrhage [67]. The most common causes of intracranial hypertension are hydrocephalus from IVH and surrounding edema from hematoma. Hence, ICP monitoring and management should be considered in patients with large hematomas or those at high risk for hydrocephalus, such as patients with a Glasgow coma scale (GCS) score of ≤8, clinical evidence of transtentorial herniation, or significant IVH or hydrocephalus [4]. ICP can be measured by catheterization into the cerebral ventricles or brain parenchyma. The decision to use these catheters should be based on whether there is a need to drain the cerebrospinal fluid to treat the hydrocephalus or elevated ICP [4]. In case of patients with hydrocephalus and decreased consciousness, ventricular catheterization and drainage is a reasonable method for ICP monitoring and management.
Methods for the medical management of ICP consist of head elevation to 30°, hyperventilation, mild sedation, and hyperosmolar therapy with hypertonic saline or mannitol. Mannitol can be administered intravenously, but special attention should be paid to volume depletion. Although hypertonic saline may require a central venous catheter, it can be more effective [68]. Corticosteroids should be avoided because of their significant harmful effects such as infections without demonstrable beneficial effects [69].
Fever and temperature control
The incidence of fever after supratentorial ICH is high, especially in patients with ventricular hemorrhage. Fever has been reported to worsen the outcomes in patients with ICH. In patients who survive the first 72 hours after hospitalization, the duration of fever is associated with poor functional outcome and seems to be an independent prognostic factor [70]. Therefore, temperature should be regularly measured in patients with ICH. Antipyretics are typically a simple method to reduce mild fever. External cooling devices and intravascular cold saline infusion can be used in different clinical settings. Preliminary studies have suggested that therapeutic cooling may reduce perihematomal edema [71,72]. Treatment with mild hypothermia in ICH should be considered investigational at this time. Although the beneficial effect of fever treatment has not been demonstrated in patients with ICH, maintenance of normothermia is reasonable and recommended in order to reduce secondary brain injury.
Glucose management
Hyperglycemia on admission is associated with an increased 28-day case fatality in both nondiabetic and diabetic patients with ICH [73]. Therefore, hyperglycemia should be controlled adequately. On the other hand, tight glucose control with intensive insulin therapy is also reported to be associated with reduced cerebral extracellular glucose availability and increased mortality [74,75]. Therefore, glucose level should be monitored regularly and both hyperglycemia and hypoglycemia should be avoided [4].
Seizure management
Studies using continuous electroencephalography (EEG) showed that electrographic seizures occurred in up to one third of the patients with ICH [76-78]. Clinical seizures are as frequent as 16% within 1 week after ICH and the location of the hematoma influences this frequency; cortical involvement is a crucial risk factor of early seizures [76,79,80]. Although the association between electrographic seizures and clinical outcomes is unclear, there is a consensus that both clinical seizures and electrographic seizures with decreased consciousness should be treated. In the case that patients with ICH presenting with decreased mental status of unknown etiology, continuous EEG monitoring is essential to detect electrographic seizures. With respect to the prophylactic use of antiepileptic drugs for ICH, there is no evidence supporting their beneficial effects. Therefore, the prophylactic use of antiepileptic drugs is not recommended [4].
Medical complications
The frequency of medical complications after ICH was reported in a randomized clinical trial of the safety of neuroprotectant use in patients with ICH. The most common medical complications were pneumonia (5.7%), pulmonary embolism (2.3%), respiratory failure (2.0%), aspiration pneumonia (2.0%), sepsis (1.3%), and urinary tract infection (0.7%) [81]. Among medical complications, pulmonary complications including pneumonia, neurogenic pulmonary edema, and pulmonary embolism were seen to be the most frequent complications. One retrospective cohort study reported that one-third of the patients with ICH developed pulmonary complications [82]. Since dysphagia and aspiration are crucial risk factors for pneumonia, formal screening for dysphagia may reduce the risk of development of pneumonia in patients with ischemic stroke [83]. Other medical complications in patients with ICH include cardiac events and death caused by acute myocardial infarction, heart failure, ventricular arrhythmias, cardiac arrest, acute kidney failure, hyponatremia, gastrointestinal bleeding, and post-stroke depression [4]. As medical complications are associated with high risk of mortality in patients with ICH usually after 7 days of hospitalization [4], comprehensive screening, monitoring, and appropriate care for each medical complication should accompany the standard management regime for ICH.
Palliative care and withdrawal of technological support
The importance of palliative care and withdrawal of technological support has recently been highlighted. The American Heart Association/American Stroke Association have published a scientific statement addressing these issues in patients with stroke [84]. The withdrawal of technological support including Do-Not-Resuscitate (DNR) orders must be considered at an individual level. There is no single accurate predictor of clinical outcomes of ICH that can be helpful in determining whether withdrawal of technological support is appropriate. Most fatalities following ICH occur within the first two days, and DNR orders are recommended to be postponed until at least the second full day of hospitalization [4]. The DNR status should not limit appropriate medical and surgical interventions, unless explicitly indicated by the patient or her/his family [4].
Surgical management
Infratentorial hemorrhage
Emergent surgery is strongly recommended in patients with a cerebellar hemorrhage with symptoms of neurological deterioration. Because the posterior fossa has little free space, cerebellar hemorrhage easily brings about brainstem compression, ventricular obstruction, hydrocephalus, and eventually high fatality. Patients with a cerebellar hemorrhage >3 cm in diameter or patients in whom cerebellar hemorrhage is causing brainstem compression or hydrocephalus can get better outcomes with surgical decompression through hematoma evacuation [4,85,86]. Initial treatment of cerebellar hemorrhage with ventricular drainage alone rather than surgical evacuation is not recommended due to insufficiency for ICP control [4].
As hematoma evacuation of a brainstem hemorrhage may be harmful in many cases, brainstem hemorrhage is usually managed conservatively [4,87,88]. Although there are several reports, which suggest that surgical treatment is effective in managing brainstem hemorrhages [89-91], the role of surgical management in treating brainstem hemorrhages remains controversial.
Supratentorial hemorrhage
The beneficial effects of surgical management of supratentorial ICH remain controversial and should be restricted in specific situations. Although several randomized trials have compared the efficacy of surgical management and conservative medical management, they have not shown significant benefits of surgical management on mortality or functional outcomes [92,93].
The International Surgical Trial in Intracerebral Hemorrhage (STICH) was conducted to prove the superiority of early hematoma evacuation (within 24 hours of randomization) over conservative medical treatment [92]. A total of 1,033 patients were enrolled from 83 centers in 27 countries, and were randomized into early surgery (n=503) or initial conservative treatment (n=530) groups. The primary outcome measure was the score on the 8-point extended Glasgow outcome scale at 6 months. Of the 468 patients randomized to the early surgery group, 26% had favorable outcomes, compared to 24% of the 496 patients randomized to the initial conservative treatment group (OR, 0.89; 95% CI, 0.66–1.19; P=0.414). The 6-month mortality rate for the early surgery group was 36%, compared with 37% for the initial conservative treatment group (OR, 0.95; 95% CI, 0.73–1.23; P=0.707). Subgroup analysis revealed that patients with lobar hemorrhages within 1 cm of the cortical surface might benefit from surgery, while patients who presented as comatose (GCS score ≤8) showed poorer outcomes following surgery. In this STICH trial, it was suggested that early surgery could be beneficial in certain patients with superficial lobar hemorrhages, but there was no overall statistically significant difference in the mortality or functional outcome between the early surgery and initial conservative treatment groups.
In 2013, the results of the STICH II trial were published [93]. The STICH II trial compared the outcomes of early surgery and initial conservative treatment in conscious patients with superficial lobar hemorrhage of 10–100 mL within 1 cm of the cortical surface, with no IVH, and who were admitted within 48 hours of the onset of symptoms. A total of 601 patients were enrolled from 78 centers in 27 countries, and were randomized into the early surgery (n=307) or initial conservative treatment (n=294) groups. The primary outcome was a prognosis-based dichotomized (favorable or unfavorable) outcome on the 8-point extended Glasgow outcome scale at 6 months. Fifty-nine percent of the patients in the early surgery group had an unfavorable outcome, compared with 62% of the patients in the initial conservative treatment group (OR, 0.86; 95% CI, 0.62–1.20; P=0.367). The 6-month mortality rate was 18% in the early surgery group and 24% in the initial conservative treatment group (OR, 0.71; 95% CI, 0.48–1.06; P=0.095) [93]. Thus, these two large randomized trials failed to prove the benefit of early surgical management with hematoma evacuation over initial conservative treatment. Hematoma evacuation might be considered as a life-saving measure in patients with supratentorial hemorrhage showing neurological deterioration [4].
Previous studies have reported that patients with a GCS score <8, significant midline shift, large hematomas, or medically intractable ICP might benefit from decompressive craniectomy [94-97]. Therefore, despite the failure of large clinical trials, it should be noted that decompressive surgery with or without hematoma evacuation might be helpful in reducing the mortality rate in these specific situations [4].
The role of minimally invasive surgical evacuation of ICH with stereotactic or endoscopic aspiration is unclear. Several studies have suggested that minimally invasive surgical evacuation might be less invasive and have better outcomes compared to a craniotomy approach [98-101]. In a randomized clinical trial conducted in China, needle aspiration of basal ganglia hemorrhages improved the 3-month functional outcome without significant improvement in mortality rate, compared to medical management alone [101]. Recently, the Minimally Invasive Surgery Plus Recombinant Tissue-Type Plasminogen Activator for ICH Evacuation Trial II (MISTIE II) reported a significant reduction in perihematomal edema in the hematoma evacuation group [100]. MISTIE III, a randomized phase 3 clinical trial, is ongoing.
Timing of decompressive surgery
On the basis of the subgroup analysis performed in the STICH II trial, surgery, if needed, should be considered within 21 hours of ictus for better outcomes [4,93]. One meta-analysis indicated that there was improved outcome with surgery if randomization was undertaken within 8 hours of ictus [102]. Another prospective study reported that surgical hematoma evacuation within 4 hours of ictus was complicated by rebleeding, indicating difficulty with hemostasis [103]. Although more evidence is needed to determine the timing of surgery, surgical management should not be delayed if patients show neurological deterioration, and surgical management can be beneficial. The possible indications for the surgical management of ICH are described in Table 3.
Intraventricular hemorrhage
IVH is usually related to deep-seated ICH in the basal ganglia and/or thalamus. IVH is a crucial determinant of poor outcomes in patients with ICH [104]. Recently, ventricular catheter insertion with thrombolytic agents has been studied to overcome the inefficiency and difficulty of maintaining catheter patency. The CLEAR-IVH trial (Clot Lysis: Evaluating Accelerated Resolution of IVH) compared treatments with recombinant tissue-type plasminogen activator (rtPA) and placebo in patients with IVH attributable to spontaneous ICH [105,106]. Patients treated with rtPA had lower ICP and less frequent ventricular obstruction. The symptomatic rebleeding rate was not significantly different between the two groups (12% in the rtPA group, 5% in the placebo group, P=0.33); the median 30-day modified Rankin scale score and mortality did not differ either. While rtPA seemed to have an acceptable safety profile in the treatment of ICH with IVH, its efficacy and safety remain uncertain [4]. Data from a well-designed phase III clinical trial, such as CLEAR III, will be needed to fully evaluate the safety and efficacy of this treatment.
Rehabilitation
Rehabilitation is strongly recommended in patients who have survived the acute stage of ICH but are discharged from acute care hospitals with disability. The main principles of rehabilitation for patients with ICH are similar to those for patients with ischemic stroke [4]. It has been repeatedly reported that the improvement of functional outcome was significantly greater in patients receiving rehabilitation compared with those receiving standard medical care [107,108]. On the basis of our knowledge about stroke recovery, rehabilitation is recommended to be started as early as possible and should be continued in the community as part of a well-coordinated program of accelerated hospital discharge and home-based resettlement to promote ongoing recovery [4].
Conclusion
In conclusion, in order to overcome the high mortality and morbidity, it is recommended that patients with spontaneous ICH should be taken care of in well-organized specialized stroke care facilities with a multidisciplinary team approach. All the management principles including the close monitoring of vital signs and neurological status, rapid and adequate BP correction, proper ICP control and timely surgical management of selected patients, prevention of complications, and early rehabilitation are important for better clinical outcomes.
Notes
The authors have no financial conflicts of interest.