Practical Issues to Prevent Stroke Associated with Non-valvular Atrial Fibrillation
Article information
Abstract
Stroke associated with non-valvular atrial fibrillation (NVAF) is one of the most important subtypes of ischemic stroke, and its importance is becoming even more apparent in an aging population. To assess the risk of stroke associated with NVAF, the CHADS2 and CHA2DS2-VASc scores are mainly used. Such scores can be used to predict the recurrence and prognosis of ischemic stroke. In addition, new oral anticoagulants (NOACs) and devices are being evaluated in the prevention of stroke associated with NVAF in addition to treatment with the conventional oral anticoagulant, warfarin. Since clinical experience with NOACs is not globally sufficient, a cautious approach is needed.
Introduction
Cardioembolic stroke, which is an important subtype of ischemic stroke, involves a large infarct volume and multiple vascular territories. The neurologic deficits are grave and develop abruptly.1,2 Because cardioembolic stroke is generally severe and frequently recurs, its long-term mortality is high.3,4 In addition, hemorrhagic transformation occurs frequently because of early or delayed recanalization.5,6 Cardioembolic stroke accounts for 14-30% of all ischemic stroke.7-12 In Korea, it accounts for approximately 17%, and the proportion is increasing (Figure 1).13,14 Cardioembolic stroke is associated with chronological age7 and is thought to be one of the most important subtypes of ischemic stroke in aged or aging populations.
Non-valvular atrial fibrillation (NVAF) is the most important cause of cardioembolic stroke, and patients with NVAF can complain of palpitations, chest pain, breathing difficulty, dizziness, or fainting.15 However, many patients do not have any symptoms or complain of vague and non-specific symptoms.16,17 Probability of stroke incidence in patients with atrial fibrillation is 3-4%,18 and the risk of stroke increased by five times in all age groups.19,20 The percentage of strokes attributable to atrial fibrillation increases steeply from 1.5% at age 50 to 59 years to 23.5% at age 80 to 89 years.21 The prevalence of atrial fibrillation is globally increasing over time. In the United States, the number of patients with atrial fibrillation was 2.1 million in 1997, but it increased to 2.3 million in 2001. It is estimated to increase to 5.6 million in 2050.22 The increased prevalence of atrial fibrillation is caused by improved survival rates of patients with heart disease.23 The prevalence of atrial fibrillation significantly increases with an increase in age.24 In Korea, 57% of individuals with atrial fibrillation are older than 65 years, and the prevalence of atrial fibrillation is the highest in those older than 80 years.25 The importance of atrial fibrillation in ischemic stroke will further increase in Korean society, which is rapidly aging.
Risk assessment of stroke in patients with NVAF
Risk factors increasing the incidence of stroke in patients with NVAF are known to be female gender, old age, history of stroke or transient ischemic attack (TIA), hypertension, heart failure, diabetes, and vascular diseases.23,26,27 History of stroke or TIA increases the risk of stroke in patients with NVAF by three times.26 The incidence of stroke in patients with NVAF in their 70s is seven times that of patients in their 40s.28 When patients with NVAF have hypertension, the risk of stroke is thrice as high.19 Since a risk stratification scale for embolic events in patients with NVAF was developed based on integration of these risk factors, it can be used to assess the risk of stroke in patients with NVAF and to select adequate preventive drugs.
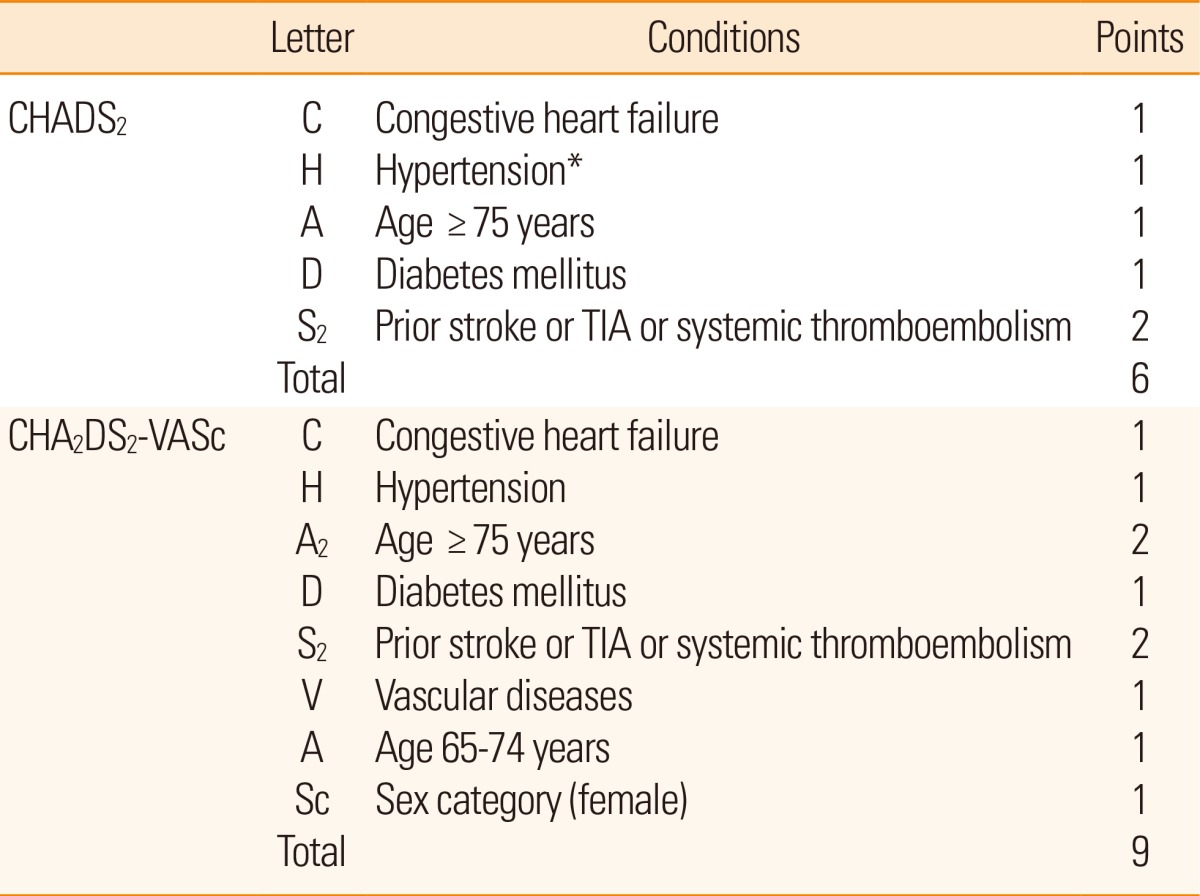
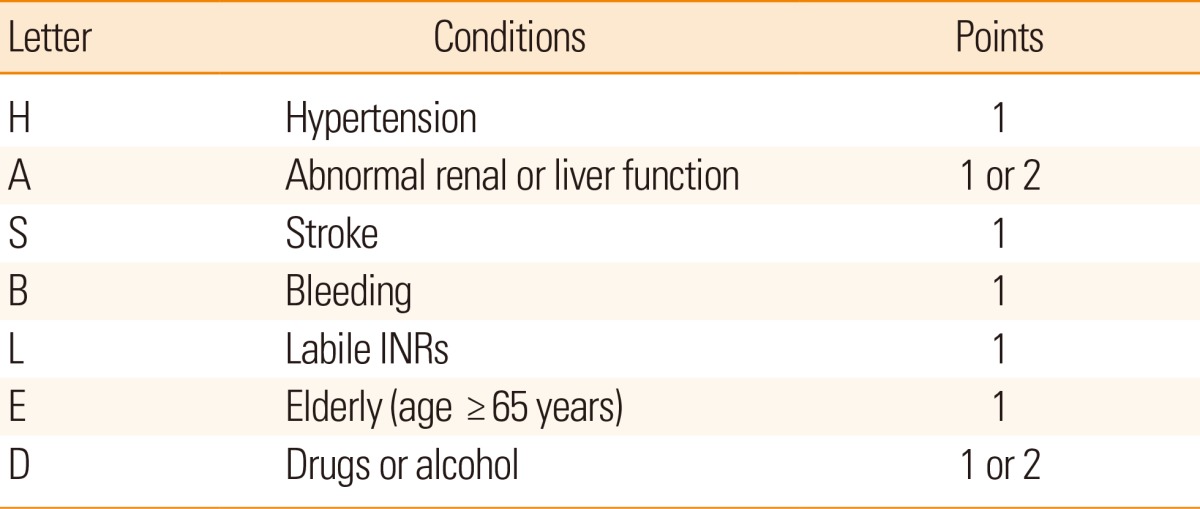
To classify the risk of stroke in patients with NVAF, several models are currently utilized. The representative stratification systems currently being used include the CHADS2 and CHA2DS2-VASc scores (Table 1).29,30 The CHADS2 score is widely used as the risk stratification scale, but after considering additional risk factors such as vascular disease, gender, and age of 65-74 years, a more specific evaluation can be made. The scale reflecting these risk factors is the CHA2DS2-VASc score. If a patient is classified into the group of low risk (0 or 1) in the point system by using the CHADS2 score, the CHA2DS2-VASc score can be helpful for a more comprehensive risk assessment.30 In addition, the HAS-BLED score, which is a convenient bleeding risk scale, is prepared from risk factors and a systematic review of bleeding in patients with NVAF (Table 2).31 If the HAS-BLED score is ≥3, a patient is classified into the high-risk group for bleeding.32 When antithrombotic therapy is started, special care and regular observation are required in this group. In addition, microbleeds predict intracranial bleeding associated with warfarin.33,34
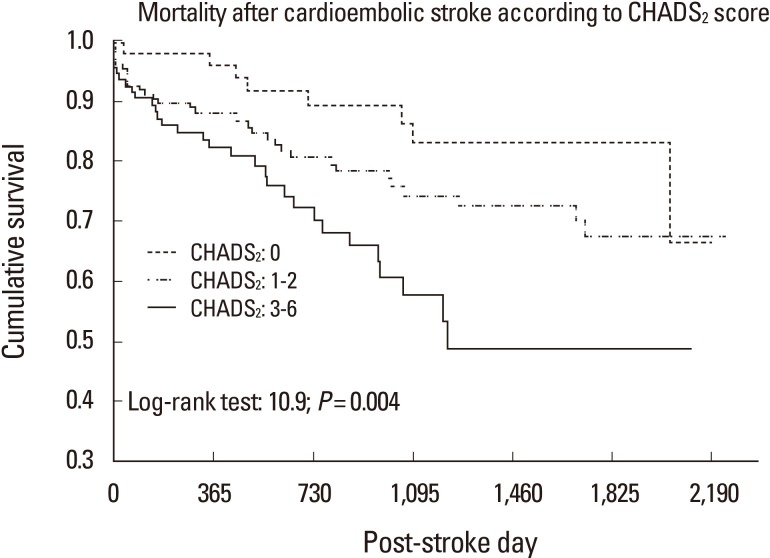
For the past 60 years, when the need for anticoagulation therapy was determined based on the calculated risk of stroke in patients with NVAF, warfarin has been administered. Previous studies demonstrated that warfarin was superior to other antiplatelet drugs in preventing stroke in patients with NVAF.35,36 In addition, it has been reported that the stroke is less severe when it occurs in patients taking warfarin.37-39 Some patients have a very high tendency of relapse stroke, and stroke may recur despite the use of warfarin after stroke associated with NVAF. Although the primary reason is that warfarin is not properly administered or the international normalized ratio (INR) does not enter the target, other risk factors for recurrence of stroke are old age, female gender, and a history of stroke.40 If large artery atherosclerosis such as carotid or vertebral atherosclerosis is associated with NVAF, the recurrence rate of stroke also increases.41 In addition, a meta-analysis reported that stroke may be more recurrent in Asian individuals taking warfarin compared to other races.42 In this context, the CHADS2 score can be useful in predicting the recurrence of stroke in patients with stroke associated with NVAF.40,42 Furthermore, it was recently reported that the CHADS2 score can be used to predict the prognosis of stroke associated with NVAF (Figure 2).43,44
Emergence of new anticoagulants
Warfarin, a vitamin K antagonist, was the only oral anticoagulant available for a long time. However, its narrow therapeutic window and numerous food and drug interactions affect its safety, efficacy, and patient compliance. In addition, its slow onset of action and variable pharmacologic effects make it difficult to maintain the appropriate antithrombotic effect.45 A meta-analysis reported that 44% of bleeding complications with warfarin were associated with supratherapeutic INRs, and 48% of thromboembolic events occurred in the subtherapeutic range.46 These drawbacks have encouraged the development of new anticoagulants. Several new oral anticoagulants (NOACs) have been developed in the past 10 years.47 Three of them were approved for use in the prevention of stroke and systemic embolism in patients with NVAF: dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, activated factor X inhibitors.48-50 They have predictable anticoagulant effects and fewer food and drug interactions, which allow a fixed dosing regimen without the need for monitoring. However, strict patient compliance is required to maintain the desired anticoagulation levels. When rapid reversal of the anticoagulant effect is needed in the event of a major bleeding or emergency surgery, there are no specific antidotes and standardized tests to monitor the anticoagulant status, which is a weakness of NOACs.51
In terms of efficacy, according to the three randomized clinical trials that compared the efficacy of three NOACs and warfarin, NOACs were not always superior to warfarin. The rates of the primary efficacy outcomes of any type of stroke or systemic embolism were significantly lower in the dabigatran 150 mg and apixaban groups compared to the warfarin group. Those in the dabigatran 110 mg and rivaroxaban groups were not significantly lower than those in the warfarin group. In addition, all three studies had poor or moderately controlled patients (defined as INR in the target range <65% of the time) as a control group.48-50 Follow-up studies in which the control groups were divided based on INR level did not show the superiority of the dabigatran 150 mg dose compared with the well-controlled group (INR in the target range ≥65% of the time).52 The superiority of the primary efficacy outcome in apixaban use was mostly caused by a reduction in hemorrhagic stroke, and there was no statistically significant difference in ischemic stroke rate.49 Therefore, if the patient is well-controlled with warfarin and has minimal complications, the use of NOACs may be controversial (Table 3).
In terms of safety, the major bleeding complication rate in the dabigatran 110 mg and apixaban groups was significantly lower than that in the warfarin group. However, there was no significant difference between the dabigatran 150 mg and rivaroxaban groups and the warfarin group (Table 3).48-50 Considering subtypes of bleeding, the intracerebral hemorrhage (ICH) rate was significantly lower with both doses of dabigatran, but the major gastrointestinal (GI) bleeding rate was significantly higher in the dabigatran 150 mg and rivaroxaban groups.50,53 The ICH rate was significantly lower and the GI bleeding rate was not significantly different in the apixaban group.49 Hemorrhagic stroke rates related to receiving warfarin were higher in Asian patients than in non-Asian patients, but hemorrhagic stroke rates were significantly reduced by dabigatran in both Asian and non-Asian patients compared with individuals receiving warfarin.54 In non-hemorrhagic adverse events, approximately 10% of the patients in the dabigatran group complained of severe dyspepsia and approximately 21% of them discontinued the drugs. These symptoms were likely caused by the tartaric acid core composed of dabigatran etexilate. Tartaric acid created an acidic environment and increased the absorption of the drug independent of gastric pH.55 Approximately 0.8% of patients taking dabigatran in the RE-LY trial experienced myocardial infarction. It was not statistically significant compared with the group taking warfarin, but it showed greater tendencies.56 A meta-analysis of 7 studies including the RE-LY trial showed that the risk of myocardial infarction and cardiac death or unstable angina was significantly increased in the dabigatran group compared with the warfarin group.57
The bioavailability of dabigatran is only 6.5% and is not influenced by the coadministration of food. Approximately 80% of the drug is excreted unchanged in the urine, while only 20% is excreted in the feces. Therefore, it is contraindicated in severe renal impairment. It is not metabolized by cytochrome P450 isoenzymes. Thus, it is substantially unaffected by mild to moderate hepatic failure.58 Because of the low plasma protein binding, it may be dialyzable when rapid reversal of its anticoagulation effects is needed.59 The bioavailability of rivaroxaban is dose-dependent and influenced by the coadministration of food. The bioavailability at 10 mg and 20 mg in the fasting state is 80%-100% and 66%, respectively. Taken with meals, its absorption is delayed but increased. Thus, therapeutic doses of rivaroxaban should be taken with meals.60 Approximately 66% is excreted in the urine, and only 28% is excreted in the feces. The safety and efficacy of rivaroxaban were consistent with warfarin in patients with moderate renal impairment, and it was also observed in Japanese patients.61 Two-thirds of the drug is converted into inactive metabolites through different CYP450 isoenzymes (CYP3A4/5 or CYP2J2) and CYP-independent mechanisms.62 In this context, rivaroxaban should be avoided in patients with severe renal failure or moderate to severe hepatic impairment. The bioavailability of apixaban is approximately 50% and is not influenced by the coadministration of food.63 One-third of the drug is metabolized through the cytochrome P-450 isoenzyme system (mainly CYP3A4/5). Approximately 25% is excreted in the urine, and >50% is excreted in the feces.64 It may be suitable for patients with mild to moderate renal impairment because of its low renal excretion.
Special circumstances for NOACs
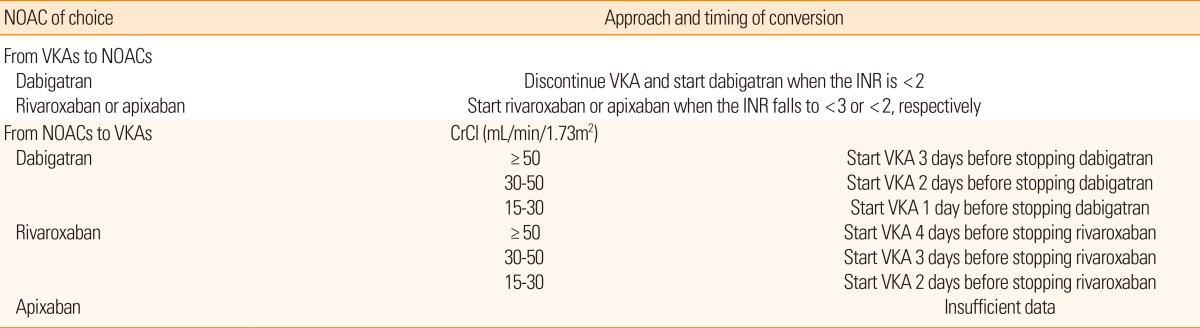
As previously mentioned, patients stabilized on warfarin may prefer to continue the same. However, the convenience and efficacy of NOACs in the patients with inadequate INR are cause to consider the transition to NOACs. Transition to warfarin also may be needed in patients who are unable to continue on NOACs. In this situation, safe transition between anticoagulants is an important issue in current practice. In previous trials including patients who had warfarin before starting on dabigatran, the highest INR permitted at the time of transition was 2.0 or 2.3,65,66 whereas in the Rivaroxaban Once-Daily, Oral, Direct Factor X Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF), it was 3.0.50 In these trials, the overlap was not associated with an increased risk for bleeding. In transition from NOACs to warfarin, it is necessary to take into account the expected onset of warfarin as well as the half-life of the NOACs. The mean time to achieve therapeutic range (INR 2-3) was approximately 5 days. The half-life of NOACs highly depends on renal function. The half-life of dabigatran varies from 14-17 hours with normal renal function to 18-27 hours in moderate to severe renal impairment.67,68 The recently recommended approaches based on these data are summarized in Table 4.69-71 The longer overlap period of rivaroxaban and warfarin shown in this table is caused by the shorter half-life of rivaroxaban.72 When assessing the INR during transitions between rivaroxaban and warfarin, we need to be cautious because rivaroxaban may increase the prothrombin time.73
Perioperative management of the NOACs is based on the urgency of the procedure, bleeding risk, and current renal function. Preoperative discontinuation of the drug is based on pharmacokinetic data and considerations regarding the bleeding risk (Table 5). Interruption of dabigatran for 48 hours should be sufficient to ensure adequate hemostasis in patients with normal renal function because of the half-life of 14-17 hours.74 For procedures with a low risk of bleeding such as cardiac catheterization, diagnostic endoscopy, and minor orthopedic surgery, for which an INR of 1.5 for patients on warfarin would be acceptable, it is reasonable to interrupt dabigatran for 24 hours. In patients with decreased renal function, the period of interruption should be longer. This principle is also applied to rivaroxaban. In the previous trial, ROCKET-AF, rivaroxaban was interrupted approximately 2 days before the procedure.60 The short period of interruption does not require bridging therapy with unfractionated heparin or low-molecular-weight heparin. Reinitiation of NOACs after the procedure depends on the postoperative risk of bleeding. For procedures with good hemostasis, the suggestion is to reinitiate NOACs at a minimum of 4-6 hours after the procedure.65 The first dose of dabigatran should be a half-dose (75 mg), and the next scheduled dose should be the usual maintenance dose. A similar strategy using a 10-mg dose for the first dose can be applied to rivaroxaban. Patients with bowel paralysis may require bridging with parenteral anticoagulants because they cannot take oral anticoagulants.
Novel strategies to prevent stroke in patients with NVAF
For strategies to prevent stroke associated with NVAF in addition to antithrombotic therapy, studies on procedures to surgically block the left atrial appendage (LAA), which is the most important position in which thrombus is formed by NVAF, have been conducted.75-77 After a device to block LAA is inserted, warfarin is administered for 45 days, and then both aspirin and clopidogrel are administered for 4.5 months. It is not inferior to the group of conventional warfarin use when aspirin monotherapy is administered.78 In addition, when long-term follow-up continues for up to 2.3 years, it is non-inferior in occurrence of cardiovascular diseases including stroke compared with the group using warfarin.79 Other strategies to prevent stroke associated with NVAF include rhythm control and heart rate control. Previous studies and meta-analyses showed that the beneficial effect between rate control and rhythm control was not different for prevention of stroke.80-85 A post-hoc analysis from a recent study showed that dronedarone, a newly developed rhythm control drug, was beneficial in preventing stroke,86 but another study using dronedarone in addition to standard therapy for rhythm control showed that dronedarone increased the risk of stroke more than two-fold in comparison with placebo.87 Recently, a large-sized population-based observational study to compare rhythm control and rate control in patients with atrial fibrillation was conducted in Canada.88 Risk for the occurrence of stroke was decreased by 20% in patients who underwent rhythm control compared with those who underwent rate control. When patients were classified into low-, moderate-, and high-risk groups based on CHADS2 scores, rhythm control was superior to rate control in the moderate- and high-risk groups. To date, a complete conclusion about the effect of rate control and rhythm control on occurrence of stroke associated with NVAF has not been made.
Conclusion
Knowledge about risk stratification and treatment for stroke associated with NVAF is increasing dramatically. More delicate risk assessment systems make physicians choose adequate preventive strategies to reduce stroke related to NVAF, and NOACs may be additional therapeutic options for these patients in primary and secondary prevention. In this context, there are some clinical practice guidelines for the management of atrial fibrillation patients from the United States and Europe.26,89,90 Recently, a clinical practice guideline for stroke prevention was published and revised in Korea, and it includes these issues about ischemic stroke associated with NVAF.91 Ischemic stroke related to NVAF has been more prevalent, as life expectancy is increasing worldwide, and disease burden is also increasing steeply. Preventive and therapeutic options should be developed and improved in the future.
Notes
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
The authors have no financial conflicts of interest.