|
 |
- Search
| J Stroke > Volume 17(2); 2015 > Article |
Abstract
Cerebral small vessel disease (SVD), which includes white matter hyperintensities (WMHs), silent brain infarction (SBI), and cerebral microbleeds (CMBs), develops in a conjunction of cumulated injuries to cerebral microvascular beds, increased permeability of blood-brain barriers, and chronic oligemia. SVD is easily detected by routine neuroimaging modalities such as brain computed tomography or magnetic resonance imaging. Research has revealed that the presence of SVD markers may increase the risk of future vascular events as well as deteriorate functional recovery and neurocognitive trajectories after stroke, and such an association could also be applied to hemorrhagic stroke survivors. Currently, the specific mechanistic processes leading to the development and manifestation of SVD risk factors are unknown, and further studies with novel methodological tools are warranted. In this review, recent studies regarding the prognostic impact of WMHs, SBI, and CMBs on stroke survivors and briefly summarize the pathophysiological concepts underlying the manifestation of cerebral SVD.
Stroke is a major cause of substantial disability and the fourth-leading cause of mortality in United States.1 In Korea, the incidence of stroke is estimated to be 216 per 100,000 person-year, and it will steeply increase as the geriatric proportion of the population increases.2 Recanalization of occluded cerebral arteries and reconstitution of brain perfusion before irreversible infarction occurs is the paramount therapeutic strategy for acute ischemic stroke. Prevention of acute deterioration, which is not uncommon in the acute stage of stroke, is also an important duty of all stroke physicians. However, the response to hyperacute revascularization treatment and acute management varies among patients. There are numerous reasons for such variability, but one plausible explanation includes individual vulnerability of the brain tissue to ischemic insults due to chronically accumulated minute injuries and the resultant decreased functional reserve capacity.
Scores of years of history for minute injuries leaves more or less a few stigmas. Well-known neuroimaging features include white matter hyperintensities (WMHs), silent brain infarction (SBI), and cerebral microbleeds (CMBs). In addition to traditional markers, perivascular space widening from localized or diffuse brain atrophy was recently suggested as one constituent of cerebral small vessel disease (SVD).3 Such signs are usually treated as chronic ones, but there should have been an acute stage of such lesions when an ischemic insult first took place. In this context, recent discussions introduced a new concept that incidentally detected acute subcortical or cortical lesions on diffusion-weighted images may indicate chronic WMHs or SBI.3
In this review, we examine how these neuroimaging markers modify the prognosis after ischemic or hemorrhagic stroke and what mechanisms exist behind such modifications. Regarding the terminology of cerebral SVDs, we use WMH, SBI, and CMB in the current review because we assume these are the most neutral ones in view of pathology and imaging.
Rapid reconstitution of cerebral blood flow through intravenous injection of alteplase or endovascular intervention is an established treatment for acute ischemic stroke patients to improve functional recovery and survival. However, this recanalization strategy is inevitably accompanied by various adverse effects, including hemorrhagic complications, in some cases.4 Cerebral SVD can aggravate immediate- and long-term outcomes after acute treatment of ischemic stroke.
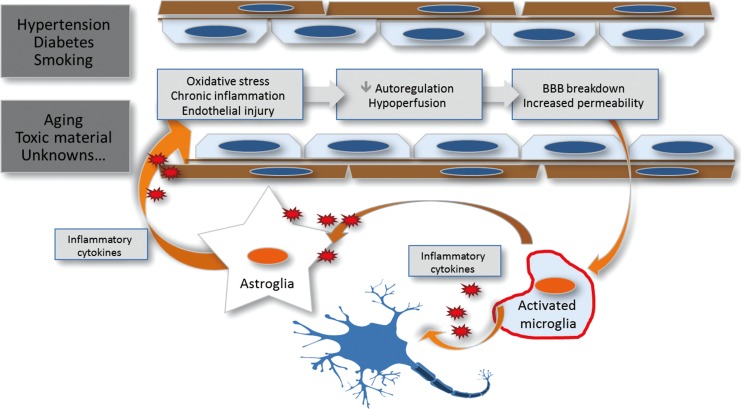
WMHs and CMBs have been gaining wide apprehension on this issue (Table 1). Advanced WMHs can increase the infarct core volume and number of irreversible lesions after major cerebral artery occlusion.5 Likewise, the extent of WMH can delay immediate neurological recovery during the acute 24-hour period after onset6 as well as functional recovery at 3 months after stroke.7 However, in an analysis of 820 intravenously treated cases in Canada during 2.5-year period, the presence of SVD markers was not associated with the overall clinical outcome at 3 months but did increase the risk of symptomatic intracranial hemorrhage.8 After endovascular recanalization treatment, an emerging but powerful alternative for intravenous alteplase, more extensive WMH was associated with an increased risk of hemorrhagic conversion and a shift in the 3-month modified Rankin scale score toward worse outcome.9,10
From the earlier research of CMBs, it was related to the pathological process resulting minute brain bleeding and thus increases the incidence of hemorrhagic stroke in patients who regularly took aspirin or warfarin.11,12 In acute stroke, a series of case reports also raised a hemorrhagic suspicion from the presence of CMB.13,14 However, a subanalysis of the DEFUSE study and a retrospective analysis based on 279 cases contradicted these findings.15,16 In a pooled analysis of 570 intravenously treated acute stroke cases from 13 centers worldwide, the incidence of symptomatic hemorrhage slightly increased by 3% in cases with coexisting CMB, but this increase was not statistically significant.17 However, the ongoing argument over this is not over yet. A meta-analysis of 5 papers that was published in 2013 reported that CMB was associated with a 2.3-fold increased odds of developing symptomatic hemorrhages after acute treatment, and the association was consistent among the included papers.18 In 2014, two new reports were published on this issue. The mere presence of CMB was again not associated with symptomatic hemorrhage after intravenous alteplase injection. However, multiple CMBs substantially increased bleeding complications in the brain, and a graded relationship with the number of CMBs was noted.19 Another group of researchers used the cutting-edge technology of susceptibility-weighted imaging and found that the presence of numerous CMBs on these images was not associated with the incidence of hemorrhagic complications.20
Currently, the presence of CMB itself is not considered to be a contraindication for hyperacute reperfusion treatment for ischemic stroke. However, considering the impact of multiple CMBs, a superfluous CMB may shift a physician's decision toward the discard of intravenous treatments. Further research is warranted. Likewise, a detailed analysis of the impact of CMB location (such as cortex, deep or infratentorial region) has not been performed.
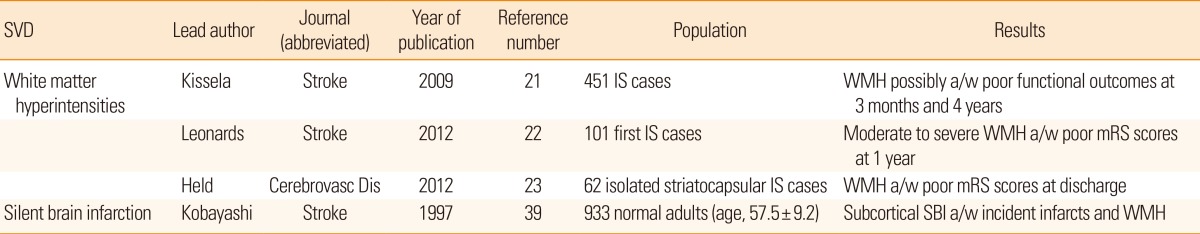
In parallel to the previous discussion, coexisting cerebral SVDs deteriorate functional recovery after stroke (Table 2). In an observational analyses of consecutive ischemic stroke patients, the extent of WMH was associated with a higher modified Rankin scale score at 3 months and 1 year after stroke on-set.21,22 WMH in striatocapsular infarction was also shown to be inversely correlated with functional recovery after removing the confounding effect of age.23 Such an association was again documented in cases with peripheral arterial stiffness without stroke history.24 Recovery from stroke is a function of the widespread neural network and its ability to compensate for the damaged structures and neural connections to adapt to a decreased functional status.25,26 However, we still do not have detailed information regarding functional outcomes after stroke in cases with SBI or CMB.
A stroke physician's main concern after hyperacute stroke treatment is the neurological and medical stabilization of the stroke patient as well as long-term secondary prevention against the recurrence of stroke or vascular events (Table 3). Traditionally, the neuroimaging markers of cerebral SVDs are known to elevate the risk of recurrent events. However, one should be cautious in interpreting these reports, because it is still difficult to make a precise distinction between the direct effects of SVD markers and bystander phenomena from shared vascular risk factors causing systemic micro- and macrovasculopathies.
WMH has been reported to increase the incidence of vascular diseases, including stroke, in a series of cohort studies based on the community-dwelling general population. In a US cohort study conducted within a healthy population with combined cerebral WMH and retinopathy, both of which are easily detectable microvascularopathies in the living, there was an 18-fold higher 5-year cumulative incidence of stroke.27 A European cohort study also reported similar findings that a healthy elderly person with SBI or WMH has a 3- to 4-fold increased risk of future stroke.28 The finding was duplicated in a community cohort study from Japan with 16-year of follow-up in which an excessive risk of mortality from SBI and WMH was reported.29 In this context, further reports have discussed the prognostic impact of cerebral SVDs including rapid deterioration of cognitive impairments.30 In a follow-up analysis from the LADIS study, the progression of WMH as well as cross-sectional status correlated with development of a new lacunar infarction.31 However, a new lacunar infarction does not imply a symptomatic one.
In parallel to the reports from the general population, a worse prognosis with cerebral SVDs was also documented in a high-risk population with established cardiovascular disease. The extent of WMH or SBI increased the risk of lacunar infarction,32 ischemic stroke and myocardial infarction,33 and mortality or ischemic stroke.34,35 CMB, in contrast to WMH or SBI, represents the bleeding tendency of an individual's brain and may behave differently from other markers in secondary prevention.36 In a pooled analysis of 3,067 ischemic stroke or transient ischemic attack patients from 10 cohort studies, patients with CMB had a consistently elevated risk of recurrent stroke, both hemorrhagic and ischemic.37 A subgroup analyses was performed according to ethnic group, and interestingly, Asian stroke survivors with CMB had a significantly higher risk of hemorrhagic stroke, but not of ischemic stroke. As discussed in the previous section, further studies involving CMBs should consider the number and location of lesions in addition to their mere presence.
SBI has been studied relatively less frequently than WMH or CMB. An interesting hypothesis was suggested recently that SBI, which usually presents as an old infarction, does not have different properties than symptomatic lacunar infarcts, and their size and location are the determining factors for their presenting symptoms.38 In light of this hypothesis, subjects with baseline SBI were reported to have a higher incidence of future stroke,39 and symptomatic lacunar infarct patients also have an increased risk of recurrent stroke.40
In terms of vascular pathology, it will be both interesting and inspiring to determine whether the prognostic impact of SVD markers, supposedly from microvasculopathies, differs according to the mechanism of ischemic stroke. Researchers from the United Kingdom tried to answer this question by analyzing a 10-year OXVASC cohort and reported that the prevalence of WMH was higher in ischemic stroke patients with small vessel occlusions, but not with other mechanisms.41 This mechanistic approach would be a practical alternative to investigate this issue, as human brain specimens are hard to collect and there is no sufficient animal model of cerebral SVDs. However, readers should be cautious for any circular reasoning in investigating SVDs in ischemic stroke mechanisms.
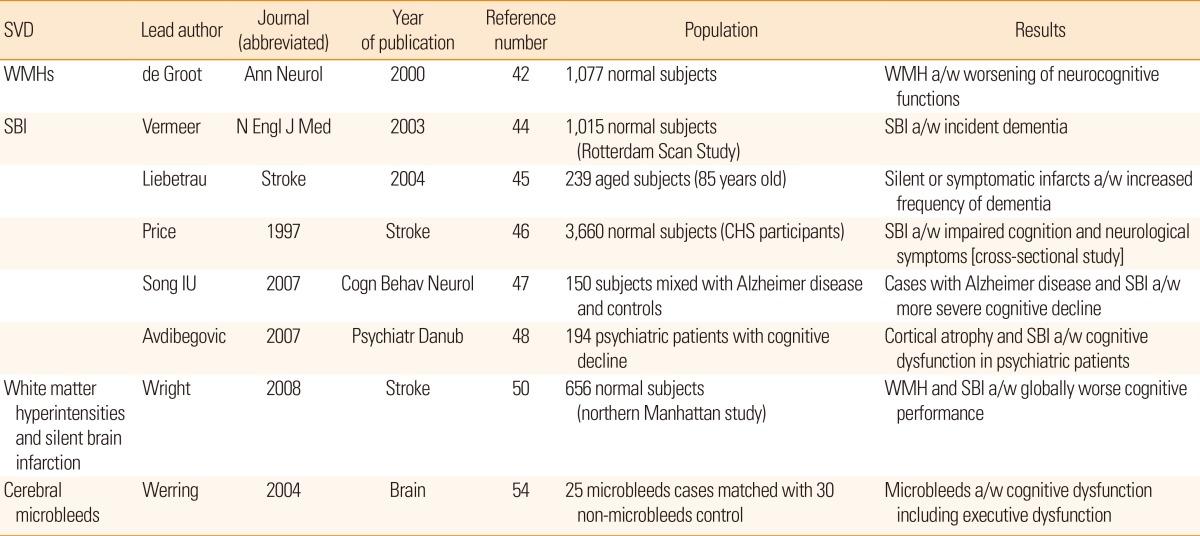
It is not hard to imagine that the long-term trajectory of cognitive functions in patients with cerebral SVDs will deteriorate, as SVD markers are neuroimaging phenomena indicating cumulative cerebral injuries (Table 4). The total volume and location of WMH is associated with worsening of neurocognitive outcomes,42 and the progression of WMH can be accompanied by a modest decline in performance on a battery of neuropsychological tests.43 The presence of SBI was associated with a 2- to 3-fold increase in the incidence of dementia44,45 and with decreased cognitive domain functioning, including visual field defects, language functions psychomotor slowing, delayed recall, and gait disturbance.46,47,48,49 Cognitive outcomes after SBI are likely to depend on the infarct location.50 Interestingly, in one report, SBI was associated with bipolar disorder with psychotic symptoms in a >50-year-old population.51 CMBs behave similarly to WMH or SBI, as they may also cause destructive lesions, 52,53 but we do not have detailed reports regarding this issue. In a small series of reports, CMBs were associated with cognitive dysfunction and gait instabilities.49,54 However, we currently do not know whether it is safe to treat CMBs equally in cases with ischemic stroke and cerebral amyloid angiopathy, as amyloid vasculopathy may intervene in the latter cases.
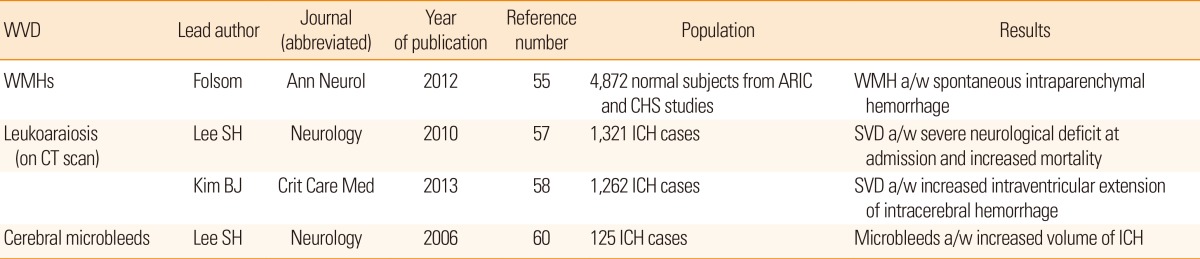
The prognostic modification of cerebral SVD markers after ischemic stroke has been duplicated in cases with hemorrhagic stroke (Table 5). In a population-based cohort study performed in the United States, the extent of WMH rated on magnetic resonance scans was associated with 71 spontaneous intraparenchymal hemorrhages during a median 13-year follow-up period, and the authors of the study also documented dose-response relationship.55 In Korea, the ABBA (Acute Brain Bleeding Analysis) study and accompanying ABBA-ICH studies recruited a large number of hemorrhagic stroke patients with both intraparenchymal hemorrhage and subarachnoid hemorrhage.56 The ABBA-ICH cohort contributed a few major findings regarding cerebral SVD and outcomes of hemorrhagic stroke, including extent of leukoaraiosis (a proxy of cerebral WMH visualized on computed tomography scans), worsening of the Glasgow Coma Scale score immediately after hemorrhagic stroke, and long-term mortality.57 Likewise, coexisting leukoaraiosis detected on computed tomography scans was associated with an increased risk of extension of hematoma into ventricles.58 Such WMHs were also inversely related to poor functional recovery after spontaneous intracerebral hemorrhage.
In the earlier period of research, CMB was highlighted in association with the aftermath following hemorrhagic stroke.59 In summary, the prevalence of CMB in patients with incident hemorrhagic stroke was estimated at 50%-70%, which is noticeably higher compared to that in ischemic stroke (20%-40%. Coexisting CMBs in spontaneous intracerebral hemorrhage cases correlated with the volume of hematoma.60 Multiple lobar CMBs, which are interpreted in terms of cerebral amyloid angiopathy, were also associated with the incidence of symptomatic lobar hemorrhage.61
Although not infrequently found and easily detected by routine neuroimaging studies, the pathophysiology of and underlying mechanisms in the development of cerebral SVD have not been elucidated in detail. Exposure to vascular risk factors, aging, and endothelial injuries initiate the pathomechanisms leading to SVD, but the detailed mechanisms of various appearances remain unknown. Peculiarity in the small vessels of the human brain is accepted as an explanation. In contrast to the usual rodent models used for biomedical research, the human brain has a relatively larger volume of white matter and the developmental process of cerebral SVD may be prolonged. Hence, current animal models do not easily mimic lacunar infarctions or the accumulation of chronic injuries.
Recent advances have allowed the opportunity to develop animal models based on rare genetic disorders to enable stroke researchers to make experimental modes for WMH, SBI, and CMB. One of the most promising models was created with substitutions of glycine residues in the collagenous domain of the Col4A1 or Col4A2 gene,62 which resulted in defects in the basement membrane of cerebral small vessels.63 When the genetically modified animal reached adulthood, spontaneous multifocal recurrent hemorrhage developed in the brain, suggesting that this may be used as an experimental model for CMB. Progress has also been made through missense mutations that alter the number of cysteine residues in the extracellular domains of NOTCH3, also known as CADASIL, which is discussed in another article in the current issue.
Prolonged exposure to aging, vascular risk factors, and unknown offenders may provoke various changes in the brain tissue and cerebral vascular beds. Such changes usually relate to structural and functional modifications of the blood-brain barrier (BBB), functionally composed of endothelial cells, with adherens junctions and tight junctions. Accumulated injuries in endothelial cells lead to structural derangement of the BBB, including the basement membrane and its barrier function, which also causes elongation of cerebral capillaries and increased permeability of the barrier.64,65 This situation would have two impacts: 1) heightened intraparenchymal inflammation from the oxidative stress caused by extravasation of blood-derived components over the BBB,66 and 2) modest but chronic cerebral hypoperfusion and a resultant decrease in cerebral protein synthesis.67 The latter would be insufficient to cause any prominent infarcts, but associated oligemic states may lead to subsequent disruption of microcirculation and additive damage to the cerebral endothelium and BBB, resulting in a positive-feedback loop of chained injuries (Figure 1).68 The overall process of endothelial injury, a permeable BBB, structural derangement of microvascular beds, parenchymal inflammation, and decreased protein synthesis will determine the individual vulnerability to future profound or minute insults from various noxious stimuli. In this context, cerebral SVD, which is a manifestation of the pathologic chain of injuries, will be a marker for individual susceptibility, but not a protagonist for the prognosis after stroke (Figure 2). Although there is a significant gap between occlusions of small arteries and extravasation of hemoglobin through the permeable BBB, the various manifestations of cerebral SVD would not make effect by their own roles but will have intertwining relations and prognostic impact for stroke survivors.
Various neuroimaging markers of cerebral SVDs, including WMHs, SBI, and CMBs, are poor prognostic markers for ischemic and hemorrhagic stroke survivors in terms of immediate response to recanalization treatment, functional recovery, recurrent vascular events, and neurocognitive trajectories. Among them, CMB has been highlighted as a predictor of future hemorrhage (as its name suggests). Until now, detailed and specific pathophysiologic information regarding the generation and manifestation of cerebral SVD has been lacking. Further studies using newly developed methodological innovations, including genetically modified animal models, proteomic investigations, or biomarker studies, are needed.69,70
References
1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:e6-e245. PMID: 23239837.



2. Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the Korean Stroke Society and Clinical Research Center for Stroke. J Stroke 2013;15:2-20. PMID: 24324935.



3. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822-838. PMID: 23867200.



4. Wahlgren N, Ahmed N, D├Īvalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) an observational study. Lancet 2007;369:275-282. PMID: 17258667.


5. Henninger N, Lin E, Haussen DC, Lehman LL, Takhtani D, Selim M, et al. Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke 2013;44:61-67. PMID: 23233384.



6. McAlpine H, Churilov L, Mitchell P, Dowling R, Teo S, Yan B. Leukoaraiosis and early neurological recovery after intravenous thrombolysis. J Stroke Cerebrovasc Dis 2014;23:2431-2436. PMID: 25174561.


7. Henninger N, Lin E, Baker SP, Wakhloo AK, Takhtani D, Moonis M. Leukoaraiosis predicts poor 90-day outcome after acute large cerebral artery occlusion. Cerebrovasc Dis 2012;33:525-531. PMID: 22538962.


8. Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM. Investigators OBOTC. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology 2007;68:1020-1024. PMID: 17389306.


9. Shi ZS, Loh Y, Liebeskind DS, Saver JL, Gonzalez NR, Tateshima S, et al. Leukoaraiosis predicts parenchymal hematoma after mechanical thrombectomy in acute ischemic stroke. Stroke 2012;43:1806-1811. PMID: 22581819.



10. Zhang J, Puri AS, Khan MA, Goddeau RP Jr, Henninger N. Leukoaraiosis predicts a poor 90-day outcome after endovascular stroke therapy. Am J Neuroradiol 2014;35:2070-2075. PMID: 24994827.



11. Wong KS, Chan YL, Liu JY, Gao S, Lam WW. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology 2003;60:511-513. PMID: 12578941.


12. Lee SH, Ryu WS, Roh JK. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology 2009;72:171-176. PMID: 19139370.


13. Kim BJ, Lee SH. Silent microbleeds and hemorrhagic conversion of an embolic infarction. J Clin Neurol 2007;3:147-149. PMID: 19513282.



14. Kidwell CS, Saver JL, Villablanca JP, Duckwiler G, Fredieu A, Gough K, et al. Magnetic resonance imaging detection of microbleeds before thrombolysis: an emerging application. Stroke 2002;33:95-98. PMID: 11779895.


15. Kakuda W, Thijs VN, Lansberg MG, Bammer R, Wechsler L, Kemp S, et al. Clinical importance of microbleeds in patients receiving IV thrombolysis. Neurology 2005;65:1175-1178. PMID: 16247042.


16. Kim HS, Lee DH, Ryu CW, Lee JH, Choi CG, Kim SJ, et al. Multiple cerebral microbleeds in hyperacute ischemic stroke: impact on prevalence and severity of early hemorrhagic transformation after thrombolytic treatment. AJR Am J Roentgenol 2006;186:1443-1449. PMID: 16632743.


17. Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, et al. Bleeding Risk Analysis in Stroke Imaging Before ThromboLysis (BRASIL): pooled analysis of T2*-weighted magnetic resonance imaging data from 570 patients. Stroke 2007;38:2738-2744. PMID: 17717319.


18. Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke 2012;8:348-356. PMID: 22973896.



19. Dannenberg S, Scheitz JF, Rozanski M, Erdur H, Brunecker P, Werring DJ, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke 2014;45:2900-2905. PMID: 25116882.


20. Gratz PP, El-Koussy M, Hsieh K, Arx von S, Mono ML, Heldner MR, et al. Preexisting cerebral microbleeds on susceptibility-weighted magnetic resonance imaging and post-thrombolysis bleeding risk in 392 patients. Stroke 2014;45:1684-1688. PMID: 24743433.


21. Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke 2009;40:530-536. PMID: 19109548.



22. Leonards CO, Ipsen N, Malzahn U, Fiebach JB, Endres M, Ebinger M. White matter lesion severity in mild acute ischemic stroke patients and functional outcome after 1 year. Stroke 2012;43:3046-3051. PMID: 22935398.


23. Held V, Szabo K, B├żzner H, Hennerici MG. Chronic small vessel disease affects clinical outcome in patients with acute striatocapsular stroke. Cerebrovasc Dis 2012;33:86-91. PMID: 22156561.


24. Lee YB, Park JH, Kim E, Kang CK, Park HM. Arterial stiffness and functional outcome in acute ischemic stroke. J Cerebrovasc Endovasc Neurosurg 2014;16:11-19. PMID: 24765608.



25. Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist 2003;9:64-75. PMID: 12580341.


26. Di Filippo M, Tozzi A, Costa C, Belcastro V, Tantucci M, Picconi B, et al. Plasticity and repair in the post-ischemic brain. Neuropharmacology 2008;55:353-362. PMID: 18359495.


27. Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Atheroslerosis Risk in Communities Study. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 2002;288:67-74. PMID: 12090864.


28. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003;34:1126-1129. PMID: 12690219.


29. Bokura H, Kobayashi S, Yamaguchi S, Iijima K, Nagai A, Toyoda G, et al. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis 2006;15:57-63. PMID: 17904049.


30. Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010;41:600-606. PMID: 20167919.



31. Gouw AA, van der Flier WM, Fazekas F, van Straaten ECW, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke 2008;39:1414-1420. PMID: 18323505.


32. Yamauchi H, Fukuda H, Oyanagi C. Significance of white matter high intensity lesions as a predictor of stroke from arteriolosclerosis. J Neurol Neurosurg Psychiatry 2002;72:576-582. PMID: 11971040.



33. Gerdes VE, Kwa VI, ten Cate H, Brandjes DP, B├╝ller HR, Stam J. Amsterdam Vescular Medicine Group. Cerebral white matter lesions predict both ischemic strokes and myocardial infarctions in patients with established atherosclerotic disease. Atherosclerosis 2006;186:166-172. PMID: 16098981.


34. Mok VC, Lau AY, Wong A, Lam WW, Chan A, Leung H, et al. Long-term prognosis of Chinese patients with a lacunar infarct associated with small vessel disease: a five-year longitudinal study. Int J Stroke 2009;4:81-88. PMID: 19383047.


35. Conijn MM, Kloppenborg RP, Algra A, Mali WP, Kappelle LJ, Vincken KL, et al. Cerebral small vessel disease and risk of death, ischemic stroke, and cardiac complications in patients with atherosclerotic disease: the Second Manifestations of ARTerial disease-Magnetic Resonance (SMART-MR) Study. Stroke 2011;42:3105-3109. PMID: 21868739.


36. Hong KS. Dual antiplatelet therapy after noncardioembolic ischemic stroke or transient ischemic attack: pros and cons. J Clin Neurol 2014;10:189PMID: 25045370.



37. Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 2013;44:995-1001. PMID: 23493732.


38. Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke 2014;45:3461-3471. PMID: 25293663.


39. Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke 1997;28:1932-1939. PMID: 9341698.


40. Putaala J, Haapaniemi E, Kurkinen M, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts, leukoaraiosis, and long-term prognosis in young ischemic stroke patients. Neurology 2011;76:1742-1749. PMID: 21576692.


41. Li L, Simoni M, K├╝ker W, Schulz UG, Christie S, Wilcock GK, et al. Population-based case-control study of white matter changes on brain imaging in transient ischemic attack and ischemic stroke. Stroke 2013;44:3063-3070. PMID: 24021688.


42. de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145-151. PMID: 10665484.


43. Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke 2007;38:2619-2625. PMID: 17673724.


44. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215-1222. PMID: 12660385.


45. Liebetrau M, Steen B, Hamann GF, Skoog I. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population-based study in 85-year-old subjects. Stroke 2004;35:1816-1820. PMID: 15205488.


46. Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue NC, Robbins J, et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. Stroke 1997;28:1158-1164. PMID: 9183343.


47. Song IU, Kim JS, Kim YI, Eah KY, Lee KS. Clinical significance of silent cerebral infarctions in patients with Alzheimer disease. Cogn Behav Neurol 2007;20:93-98. PMID: 17558252.


48. Avdibegovi─ć E, Be─ćirovi─ć E, Selimbasi─ć Z, Hasanovi─ć M, Sinanovi─ć O. Cerebral cortical atrophy and silent brain infarcts in psychiatric patients. Psychiatr Danub 2007;19:49-55. PMID: 17603416.

49. Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, et al. Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke 2012;43:1505-1510. PMID: 22442168.


50. Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, et al. White Matter Hyperintensities and Subclinical Infarction: associations With Psychomotor Speed and Cognitive Flexibility. Stroke 2008;39:800-805. PMID: 18258844.



51. Fujikawa T, Yamawaki S, Touhouda Y. Silent Cerebral Infarctions in Patients With Late-Onset Mania. Stroke 1995;26:946-949. PMID: 7762043.


52. Watanabe A, Kobashi T. Lateral gaze disturbance due to cerebral microbleed in the medial lemniscus in the mid-pontine region: a case report. Neuroradiology 2005;47:908-911. PMID: 16142477.


53. Fisher M, French S, Ji P, Kim RC. Cerebral microbleeds in the elderly: a pathological analysis. Stroke 2010;41:2782-2785. PMID: 21030702.



54. Werring DJ. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 2004;127:2265-2275. PMID: 15282216.


55. Folsom AR, Yatsuya H, Mosley TH, Psaty BM, Longstreth WT. Risk of intraparenchymal hemorrhage with magnetic resonance imaging-defined leukoaraiosis and brain infarcts. Ann Neurol 2012;71:552-559. PMID: 22522444.



56. Yoon BW, Bae HJ, Hong KS, Lee SM, Park BJ, Yu KH, et al. Phenylpropanolamine contained in cold remedies and risk of hemorrhagic stroke. Neurology 2007;68:146-149. PMID: 17210897.


57. Lee SH, Kim BJ, Ryu WS, Kim CK, Kim N, Park BJ, et al. White matter lesions and poor outcome after intracerebral hemorrhage: a nationwide cohort study. Neurology 2010;74:1502-1510. PMID: 20458066.


58. Kim BJ, Lee SH, Ryu WS, Kim CK, Chung JW, Kim D, et al. Extents of white matter lesions and increased intraventricular extension of intracerebral hemorrhage. Crit Care Med 2013;41:1325-1331. PMID: 23388516.


59. Kim BJ, Lee SH. Cerebral microbleeds: their associated factors, radiologic findings, and clinical implications. J Stroke 2013;15:153PMID: 24396809.



60. Lee SH, Kim BJ, Roh JK. Silent microbleeds are associated with volume of primary intracerebral hemorrhage. Neurology 2006;66:430-432. PMID: 16476948.


61. Van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke 2014;45:2280-2285. PMID: 24947286.



62. Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Human Mol Genet 2012;21:R97-R110. PMID: 22914737.



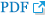
63. Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med 2006;354:1489-1496. PMID: 16598045.


64. Chen ZL, Yao Y, Norris EH, Kruyer A, Jno-Charles O, Akhmerov A, et al. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J Cell Biol 2013;202:381-395. PMID: 23857767.



65. Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J 2010;74:608-616. PMID: 20234102.



66. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013;62:810-817. PMID: 23980072.



67. Wei EP, Kontos HA. Increased venous pressure causes myogenic constriction of cerebral arterioles during local hyperoxia. Circ Res 1984;55:249-252. PMID: 6744533.


68. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008;57:178-201. PMID: 18215617.


69. Datta A, Chen CP, Sze SK. Discovery of prognostic biomarker candidates of lacunar infarction by quantitative proteomics of microvesicles enriched plasma. PLoS One 2014;9:e94663PMID: 24752076.



70. Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J. Blood markers of coagulation, fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke: systematic review and meta-analysis. Cerebrovasc Dis 2014;37:64-75. PMID: 24401164.


Figure┬Ā1
Prolonged exposure to aging, vascular risk factors, and unknown offenders may provoke various changes in the brain tissue and cerebral vascular bed, including basement membrane and blood-brain barrier function, which also causes elongation of cerebral capillaries and increased permeability of the barrier. This leads to heightened intraparenchymal inflammation due to the oxidative stress caused by extravasation of blood-derived components over the BBB, modest but chronic cerebral hypoperfusion, and a resultant decrease in cerebral protein synthesis.
Figure┬Ā2
The overall process of endothelial injury, permeable BBB, structural derangement of microvascular beds, parenchymal inflammation, and decreased protein synthesis will determine the individual vulnerability to future profound or minute insults from various noxious stimuli. Cerebral SVD, a manifestation of the pathologic chain of injuries, will have its role as a marker for individual susceptibility.

Table┬Ā1
Prognostic impact of cerebral small vessel diseases (SVD) in acute treatment of ischemic stroke
Table┬Ā2
Prognostic impact of cerebral small vessel diseases on the functional recovery after stroke