Tenecteplase Versus Alteplase in Medium Vessel Occlusion Ischemic Stroke: A Secondary Analysis of the Alteplase Compared to Tenecteplase Randomized Trial
Article information
Abstract
Background and Purpose
The safety and efficacy of tenecteplase in patients with ischemic stroke due to medium vessel occlusion (MeVO) are not well studied. We aimed to compare tenecteplase with alteplase in stroke due to MeVO.
Methods
Patients with baseline M2-middle cerebral artery (MCA), M3/M4-MCA, P2/P3/P4-posterior cerebral artery (PCA), A2/A3/A4-anterior cerebral artery (ACA) occlusions from the Alteplase Compared to Tenecteplase (AcT) trial were included. Primary outcome was the proportion of 90-day modified Rankin Scale (mRS) 0–1. Secondary outcomes were 90-day mRS 0–2, ordinal mRS, mortality, quality of life measures (EuroQol 5-Dimension 5-Level, EuroQol visual analog scale), and symptomatic intracerebral hemorrhage (sICH). Initial and final successful reperfusion were reported in patients undergoing endovascular thrombectomy (EVT).
Results
Among 1,558 patients with available baseline computed tomography angiography; 455 (29.2%) had MeVO of which 27.5% (125/455) were proximal M2; 16.3% (74/455) were distal M2; 35.2% (160/455) were M3/M4; 7.5% (34/455) were A2/A3/A4; and 13.6% (62/455) were P2/P3/P4 occlusions. EVT was performed in 87/455 (19.1%) patients. mRS 0–1 at 90 days was achieved in 37.9% in the tenecteplase versus 34.7% in the alteplase group (adjusted risk ratio [aRR] 1.07; 95% confidence interval [CI] 0.91–1.25). Rates of 90-day mRS 0–2, sICH, and mortality were similar in both groups. No statistical difference was noted in initial successful reperfusion rates (13.0% vs. 7.5%) among the 87 patients who underwent endovascular thrombectomy. However, final successful reperfusion was higher in the tenecteplase group (71.7% vs. 60.0%, aRR 1.29, 95% CI 1.04–1.61).
Conclusion
Intravenous tenecteplase had comparable safety, functional outcomes and quality of life compared to intravenous alteplase among patients with MeVO. Among those treated with EVT, tenecteplase was associated with higher successful reperfusion rates than alteplase.
Introduction
Medium vessel occlusions (MeVO) block medium-sized and distal arteries with a diameter between 0.75 mm and 2.00 mm [1]. These occlusions account for up to 40% of acute ischemic stroke (AIS) and can lead to significant disability and mortality if left untreated [1,2].
Previous studies have shown high recanalization rates with intravenous thrombolysis (IVT) using alteplase in patients with MeVO compared to large vessel occlusion [2,3]. In the INTERRSeCT study (Identifying New Approaches to Optimize Thrombus Characterization for Predicting Early Recanalization and Reperfusion With IV Alteplase and Other Treatments Using Serial CT Angiography), successful recanalization (revised Arterial Occlusive Lesion 2b/3) with alteplase assessed on repeat computed tomography angiography (CTA) or on first angiographic run was seen in 37.1% patients with M2-middle cerebral artery (MCA) occlusions and in 42.5% patients with more distal occlusions (M3-MCA, anterior cerebral artery [ACA], and posterior cerebral artery [PCA]) [3].
Tenecteplase is a second-generation thrombolytic drug, with greater fibrin specificity and longer half-life than alteplase. The EXTEND-IA TNK part 1 (Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke) was a phase II trial which reported higher recanalization rates compared to alteplase for large vessel occlusion while a sub-group analysis of the phase III Alteplase Compared to Tenecteplase (AcT) trial reported similar recanalization rates [4,5]. Recanalization rates with tenecteplase may however be higher with more distal occlusion location [5,6]. In a recent post hoc analysis of the EXTEND-IA TNK trials, the authors reported higher reperfusion rates on the initial angiographic run of endovascular thrombectomy (EVT) in 30% patients with distal M1-MCA or proximal large M2-MCA vessel occlusions who received tenecteplase compared to 10% patients who received alteplase (30% vs. 10%) [6]. Data regarding recanalization, clinical and safety outcomes of tenecteplase in MeVO patients are lacking. Thus, we compared the safety and efficacy of intravenous tenecteplase (0.25 mg/kg) versus intravenous alteplase (0.9 mg/kg) in acute stroke due to MeVO presenting within 4.5 hours from symptom onset from the AcT randomized controlled trial [7].
Methods
Study design and patient population
This is a secondary analysis of the AcT trial (https://www.clinicaltrials.gov; unique identifier: NCT03889249), a phase III, pragmatic, randomized, controlled, open-label registry-linked trial with blinded outcome assessment, assessing noninferiority of intravenous tenecteplase as compared to alteplase in patients with acute ischemic stroke eligible for IVT [7].
The methods and results of this trial have been previously described [7,8]. Briefly, adult AIS patients eligible for IVT, i.e., age ≥18 years with a disabling neurological deficit within 4.5 hours of symptom onset were included from 22 Canadian stroke centers between December 2019 and January 2022. In total, 1,600 patients were randomly assigned (1:1) to intravenous tenecteplase (0.25 mg/kg) or intravenous alteplase (0.9 mg/kg). Of these, 1,577 patients who consented were included in the intention-to-treat analysis.
For this secondary analysis, we included patients with anterior or posterior circulation MeVO detected on baseline CTA. MeVO was defined as occlusion of the proximal M2-MCA segment (non-dominant or co-dominant branches), any distal M2-MCA segment, M3/M4 MCA segments, A2 to A4 ACA segments, and P2 to P4 PCA segments (Supplementary Table 1). Occlusion of proximal and dominant M2-MCA branches was considered a large vessel occlusion and was excluded from this analysis.
Indications for IVT and EVT followed the Canadian Stroke Best Practice Recommendations (CSBPR 2018) [9]. The trial was regulated by Health Canada and approved by research ethics boards at each enrolling center. The trial used deferred consent procedures wherever approved by local research ethics boards [10]. This study follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Imaging analysis
Baseline non-contrast computed tomography (NCCT), CTA, digital subtraction angiography, and follow-up images were read by an independent imaging core laboratory from the AcT trial (FBa, NS, IA, FBe, BKM, MA), blinded to treatment allocation and clinical outcomes. Baseline imaging readers were also blinded to follow-up images.
MeVO occlusion location was determined on baseline CTA. The M2 segment was defined as the segment starting from the first bifurcation of the proximal MCA excluding the anterior temporal branch and ending at the circular sulcus of the insula [11]. Further, the occluded M2 branch was divided into proximal and distal occlusion with respect to the mid-Sylvian point on coronal images, and into dominant (>50% of MCA territory) versus co-dominant (50% of MCA territory) versus non-dominant (<50% of MCA territory) occlusions [12]. Detailed definition of MeVO segments are provided in Supplementary Table 1.
In patients treated with EVT, reperfusion grade was assessed on the first and final angiographic images using the expanded Thrombolysis in Cerebral Infarction (eTICI) [13,14]. Recanalization of intracranial occlusions was assessed using the revised arterial occlusion lesion (rAOL) scale on the first angiographic run of EVT [3].
Intracranial hemorrhage was assessed on follow-up imaging at 24 hours after thrombolysis administration and was classified using the Heidelberg classification [15].
Outcomes measures
The primary outcome was modified Rankin Scale (mRS) score of 0–1 assessed at 90–120 days after randomization through standardized central telephone interviews by blinded trained research coordinators using the Rankin Focused Assessment [16].
Secondary outcomes were mRS score of 0–2, change along the full mRS scale at 90 days, length of hospital stay, return to baseline function, proportion of patients receiving EVT, successful recanalization (rAOL score of 2b-3), and successful reperfusion (eTICI score 2b-3) at first and final angiographic images, and final excellent reperfusion (eTICI score 2c-3) in patients who underwent EVT.
With more distal occlusions, e.g., patients with MeVOs, the assessment of post-stroke disability using a scale such as the mRS is less sensitive to patient disability than when patients have large vessel occlusions. This is likely because of symptom heterogeneity due to differences in affected arterial territory and brain eloquence [1]. Thus, health-related quality of life at 90 days using the EuroQuol 5-Dimension 5-Level (EQ-5D-5L) and a visual analog scale (EQ-VAS) was also assessed in addition. The EQ-5D-5L is a standardized measure of health quality and comprises 5 dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Each dimension has 5 levels of severity: no problems, slight problems, moderate problems, severe problems, and extreme problems [17,18]. The scores were combined to form a 5-digit health state and then converted into a single utility score value using the time trade-off algorithm for Canada [19]. Scores range between -1 and 1, with higher values indicating a better life. For the EQ-VAS, participants were asked to self-rate their health state on scale of 0 (worst possible quality of life) to 100 (best possible quality of life). Patients who died at 90 days were assigned an EQ-5D score of 0.
Safety outcomes were 90-day all-cause mortality, imaging identified intracranial hemorrhage and symptomatic intracerebral hemorrhage (ICH) defined as any intracerebral hemorrhage that was temporally related to and directly responsible for worsening of the patient’s neurological condition and in the investigator’s opinion was the most important factor for the neurological worsening. The safety outcomes were reported in patients who received any dose of either lytic agent and who were reported as treated.
Statistical analyses
Baseline clinical and imaging characteristics were compared between tenecteplase and alteplase groups using descriptive statistics. Categorical variables were expressed as numbers and proportions and quantitative variables as median (interquartile range, IQR). Fisher’s exact test was used for categorical data and Wilcoxon rank sum test for continuous variables. Mixed-effects Poisson regression, mixed-effects ordinal logistic regression, and mixed-effects linear regression were performed to assess the association between thrombolysis treatment type and the study outcomes (binary, ordinal, and continuous outcomes, respectively). Adjustments were made for the same confounders as in the main AcT trial, i.e., age, sex, National Institutes of Health Stroke Scale (NIHSS) score, and time from stroke symptom onset to needle [7]. The enrolling site was included as a random-effects variable to account for clustering within sites. For angiographic imaging outcomes, adjustment was limited to age and occlusion location at baseline because of the low number of events for these outcomes. Effect size estimates were reported as adjusted risk ratios (aRRs), adjusted common odds ratios or adjusted beta coefficients with their 95% confidence intervals (CI).
Further, the effect of thrombolysis treatment type in each occlusion location subgroup was analyzed using descriptive statistics. The interaction between thrombolytic type and site of occlusion for all outcomes was assessed using multiplicative interaction terms in the regression models. To avoid over stratification and small numbers, we combined M3/M4-MCA, A2 to A4 ACA, and P2 to P4 PCA occlusions into one subgroup for the interaction analyses of angiographic outcomes. No correction for multiple testing was done as all analyses were considered exploratory. No imputation was performed as missing data were minimal. All reported P-values were two-sided, with two-sided P<0.05 considered statistically significant. All analyses were performed using Stata/MP Ver 17.0 (StataCorp LLC., College Station, TX, USA).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Patient characteristics
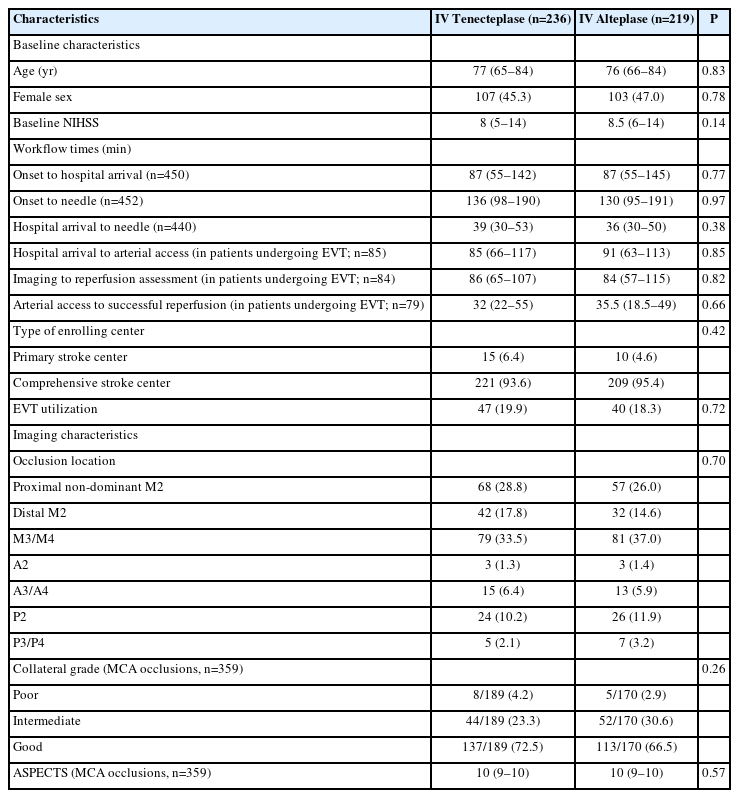
Among 1,577 patients enrolled in AcT, 1,558 had available CTA on baseline, 455/1,558 (29.2%) patients had MeVO, 236/455 (51.9%) received intravenous tenecteplase and 219/455 (48.1%) intravenous alteplase (Figure 1). Median age was 76 years (IQR 66–84 years) and 210 (46.1%) were females.

Study flowchart. AcT, Alteplase Compared to Tenecteplase; ICA, internal carotid artery; CTA, computed tomography angiography; IVT, intravenous thrombolysis; EVT, endovascular thrombectomy.
Among the 199 M2 occlusion patients, 125 (62.8%) were proximal and 74 (37.2%) were distal. Among the 125 proximal occlusion patients, 74 (59.2%) were co-dominant and 51 (40.8%) were non-dominant. Anterior division was occluded in 98 (49.2%) versus posterior division in 101 (50.8%) patients. Baseline clinical and imaging characteristics including other occlusion locations were well balanced between tenecteplase and alteplase patients. All workflow times were comparable between thrombolytic drug types, notably hospital arrival to needle (39 min [IQR 30–53] vs. 36 min [IQR 30–50], P=0.38) and hospital arrival to arterial access time (85 min [IQR 66–117] vs. 91 min [IQR 63–113], P=0.85) in tenecteplase and alteplase patients, respectively (Table 1).
Clinical and angiographic outcomes
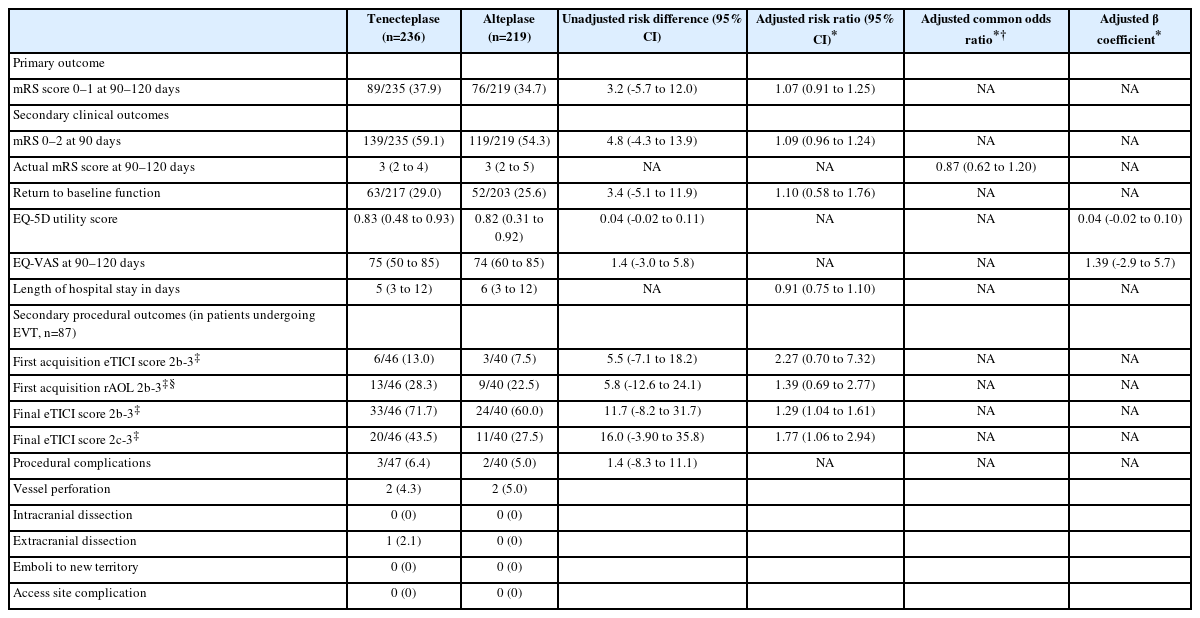
The primary outcome (mRS 0–1 at 90 days) was achieved in 89/235 (37.9%) tenecteplase patients versus 76/219 (34.7%) alteplase patients (aRR 1.07, 95% CI, 0.91–1.25, P=0.43) (Table 2 and Figure 2).
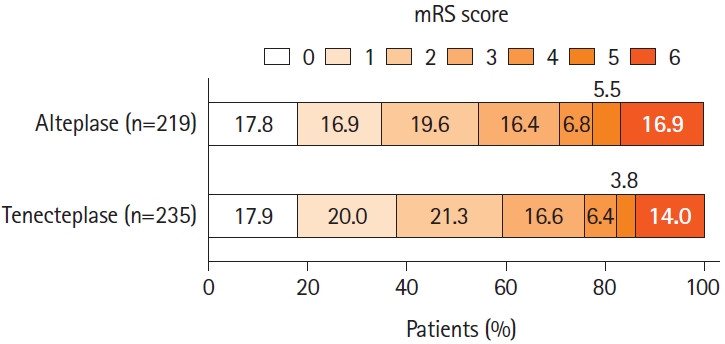
Distribution of the modified Rankin Scale (mRS) scores at 90 days. No significant difference was seen between the tenecteplase and alteplase groups in the ordinal analysis of the mRS score, adjusted for age, sex, baseline stroke severity, symptom onset-to-needle as fixed-effects variables, and site as a random-effects variable (adjusted common odds ratio, 0.87 [0.62 to 1.20], P=0.39). The mRS score ranges from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death.
There was no difference in the rates of 90-day mRS 0–2 (139/235 [59.1%] vs. 119/219 [54.3%]), return to baseline function (63/217 [29.0%] vs. 52/203 [25.6%]), median EQ-5D utility score (0.83 [IQR 0.48–0.93] vs. 0.82 [IQR 0.31–0.92]), and median EQ-VAS (75 [IQR 50–85] vs. 74 [IQR 60–85]) at 90 days between thrombolysis treatment groups (Table 2).
In the analysis of EQ-5D-5L domains, tenecteplase administration resulted in slight numerical improvements within all domains compared to alteplase (Supplementary Figures 1-5).
EVT was performed in 87 patients: 59/125 (47.2%) with proximal M2, 16/74 (21.6%) with distal M2, 4/160 (2.5%) with M3/M4, 3/34 (8.8%) with A2/A3/A4 and 5/62 (8.1%) with P2/P3/P4 occlusions. Baseline characteristics were balanced between the two thrombolytic groups (Supplementary Table 2). Angiographic outcomes were assessed in 86 patients as catheterization of proximal supra-aortic vessels was not successful in one patient. Successful recanalization (rAOL 2b-3) and successful reperfusion (eTICI 2b-3) on first angiographic run were similar between tenecteplase and alteplase groups (13.0% vs. 7.5%) among the 87 patients who underwent endovascular thrombectomy. However, successful reperfusion (33/46 [71.7%] vs. 24/40 [60.0%]) and excellent reperfusion (20/46 [43.5%] vs. 11/40 [27.5%]) on final angiography were significantly higher in the tenecteplase group (adjusted RR 1.29 [1.04–1.61, P=0.02] for successful reperfusion and 1.77 [95% CI, 1.06–2.94, P=0.03] for excellent reperfusion on final angiography). Intraprocedural vessel perforation occurred in 4 patients, 2 in each treatment group (Table 2).
Safety outcomes
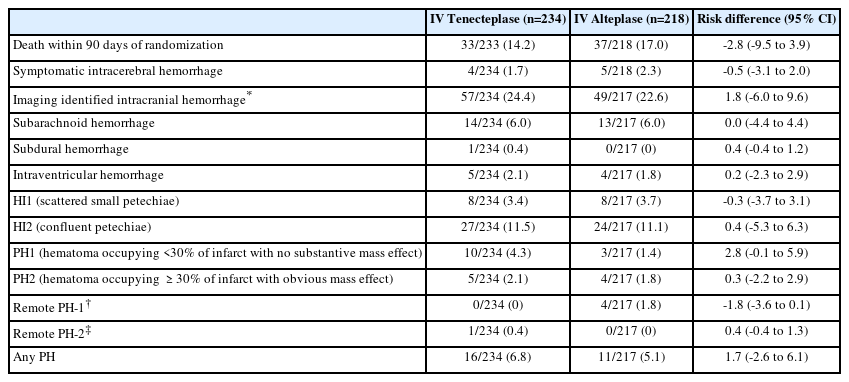
Safety was examined in 442 patients and was comparable between both treatment groups. Death within 90 days occurred in 33/233 (14.2%) versus 37/218 (17.0%) patients (unadjusted risk difference -2.8 [95% CI, -9.5 to 3.9], P=0.44). Symptomatic ICH occurred in 4/234 (1.7%) versus 5/218 (2.3%) patients (P=0.74) in the tenecteplase and alteplase groups, respectively (Table 3). Rates of any hemorrhage seen on imaging were 57/234 (24.4%) versus 49/217 (22.6%) (P=0.74) and rates of any parenchymal hematoma were 16/234 (6.8%) versus 11/217 (5.1%) (P=0.55). Details of intracranial hemorrhage subtypes as per Heidelberg classification by thrombolytic agent is provided in Table 3.
Effect modification of the relationship between thrombolysis treatment type and outcomes by occlusion location
There was no modification of treatment effect by occlusion location for all outcomes (all P interaction >0.05) (Supplementary Figure 6). The rate of mRS 0–1 at 90 days was 42.6% versus 40.3% in proximal M2-MCA occlusions (P=0.85), 33.3% versus 31.2% in distal M2-MCA occlusion (P=0.99), 41.0% versus 34.6% in M3/M4-MCA occlusion (P=0.42), 22.2% versus 25.0% in A2/A3/A4-ACA occlusion (P=0.99), and 34.5% versus 33.3% in P2/P3/P4-PCA occlusion (P=0.99) in the tenecteplase and alteplase group, respectively. Further details of clinical, angiographic, and safety outcomes by thrombolysis agent for each occlusion location is provided in Supplementary Table 3.
Discussion
Acute ischemic stroke due to MeVO was observed in nearly a third of patients in the phase III AcT randomized controlled trial. MeVO patients who received intravenous tenecteplase achieved similar clinical and safety outcomes compared to patients who received alteplase. In patients treated with EVT, initial recanalization and reperfusion rates were similar but tenecteplase was associated with better final reperfusion rates compared to alteplase (adjusted risk ratio of 1.29 for eTICI 2b/3 and 1.77 for eTICI 2c/3). This is among the first studies comparing the outcomes of MeVO patients treated with tenecteplase compared to alteplase. Previous trials of tenecteplase in stroke patients either were restricted to LVO patients or were small and reported outcomes in limited numbers (Supplementary Table 4) [5,20-29].
We found similar functional outcomes in tenecteplase- and alteplase-treated patients with MeVO in line with the results of prior tenecteplase trials [24,26]. Rates of mRS 0–1 and mRS 0–2 at 90 days in patients with M2-MCA occlusions in this study are higher than those seen in patients with M2-MCA occlusions from the control group in the HERMES (Highly Effective Reperfusion evaluated in Multiple Endovascular Stroke Trials) collaboration. These differences are likely explained by the predominance of proximal and dominant M2 occlusions in the latter study [12]. In patients with P2 to P4/PCA occlusions, functional outcomes were slightly worse in this study compared to the control group in TOPMOST (Treatment for Primary Medium Vessel Occlusion Stroke) substudy [30]. This difference might be explained by higher baseline stroke severity in this study. However, rates of functional outcomes in A2 to A4 ACA occlusions in our study are similar to those reported in a substudy of TOPMOST [31].
In line with previous trials, tenecteplase showed similar rates of symptomatic ICH, 90 days mortality, and ICH grades on imaging compared to alteplase. Rates of symptomatic ICH and mortality were similar to previous trials of tenecteplase at 0.25 mg/kg dose [32].
EVT was performed in 19% of MeVO patients in this study similar to what was reported in the pooled analysis of INTERRSeCT and PRoveIT (Precise and Rapid assessment of collaterals using multi-phase CTA in the triage of patients with acute ischemic stroke for IA Therapy) studies (21%) [2]. Rates of successful recanalization and reperfusion on first angiographic images were numerically higher in the tenecteplase group but did not reach statistical significance. These rates were lower compared to the INTERRSeCT and PRoveIT studies (47% of successful recanalization in those studies compared to 26% in this study). This may be explained by the shorter time from baseline CTA imaging to first angiographic images in this study (median [IQR]: 86 [60–111] min vs. 287 [226–382] min) as the likelihood of recanalization with alteplase and tenecteplase increases with time [3]. A prior observational study reported similar early recanalization rates before EVT between the tenecteplase and alteplase groups in patients transferred from primary stroke centers and included 28% of patients with M2 occlusions [33]. Interestingly, tenecteplase was associated with better final reperfusion compared to alteplase in our study. These findings are corroborated by the results of a prior study that reported higher early reperfusion rates with tenecteplase versus alteplase in distal M1-MCA and M2-MCA occlusions compared to ICA and proximal M1-MCA occlusions and in low clot burden occlusions compared to high clot burden occlusions [6].
Endovascular thrombectomy is challenging in patients with MeVO owing to the distal location and small diameter of occluded arteries. In our study, only 19% of MeVO patients underwent EVT and 91% were in the MCA territory, which reflects current practices in the Canadian setting. Final successful reperfusion was seen in 51/78 (65.4%) of the MCA-MeVOs which is similar to 59.2% found in the pooled analysis of 7 randomized trials from HERMES. Similarly, rates of 90-day mRS 0–2 were comparable (63.3% vs. 58.2% in HERMES) [12]. Regarding EVT safety, only 4 patients (4.6%) had vessel perforation and none had emboli to new territory. Ongoing distal occlusion trials (ESCAPE-MeVO [EndovaSCular TreAtment to imProve outcomEs for Medium Vessel Occlusions, ClinicalTrials.gov Identifier: NCT05151172], DISTAL [EnDovascular Therapy Plus Best Medical Treatment (BMT) Versus BMT Alone for MedIum VeSsel Occlusion sTroke, ClinicalTrials.gov Identifier: NCT05029414], DISCOUNT [Evaluation of Mechanical Thrombectomy in Acute Ischemic Stroke Related to a Distal Arterial Occlusion, ClinicalTrials.gov Identifier: NCT05030142], DISTALS [Distal Ischemic Stroke Treatment With Adjustable Low-profile Stentriever, ClinicalTrials.gov Identifier: NCT05152524], ORIENTAL-MeVO [Evaluation of Endovascular Treatment in Acute Intracranial Distal Medium Vessel Occlusion Stroke, ClinicalTrials.gov Identifier: NCT06146790], and FRONTIER-AP [randomized controlled trial of the clinical outcome and safety of endovascular versus standard medical therapy for stroke with medium sized vessel occlusion, ANZCTR registration identifier: ACTRN 12621001746820p]) will provide high-level data on the safety and benefit of EVT in MeVOs.
Occlusion location did not modify the effect of thrombolysis treatment on any outcome in this study. There was however a suggestion of benefit in favor of tenecteplase versus alteplase in M3/M4 versus distal M2 versus proximal M2 occlusions for the outcomes of mRS 0–1 and 0–2 at 90 days. Similar results were also noted for final reperfusion rates in distal versus proximal M2 occlusions. Further studies are needed to confirm whether tenecteplase is superior to alteplase especially in more distal occlusions.
Our study comprises a large and inclusive sample of MeVO patients from a large pragmatic randomized controlled trial and provides evidence on the safety and efficacy of tenecteplase compared to alteplase using detailed assessment of functional and quality of life outcomes. There are however a few limitations. First, this is a post hoc analysis of a phase III randomized trial with a relatively small number of distal ACA and PCA occlusions. However, this is arguably the largest study to date characterizing and comparing MeVO patients receiving tenecteplase versus alteplase (Supplementary Table 4). Moreover, baseline and imaging characteristics were well balanced between treatment groups. Second, assessment of thrombolysis-induced recanalization was only available in the minority of patients who underwent EVT. In this pragmatic trial, the decision to proceed with EVT was left to the judgment of the treating physicians. Such patients presumably had more disabling neurological deficits and/or accessible occlusions and may have confounded estimation of recanalization rates. In addition, our finding of better final reperfusion with tenecteplase in the EVT subgroup should be interpreted with caution because of the small sample size. Third, mRS score may not capture subtle functional impairment in MeVO patients. To circumvent this limitation, we used other measures such as return to baseline function, EQ-5D-5L utility score, and EQ-VAS. Moreover, the mRS is a validated measure of stroke outcome that is being used in the ongoing MeVOs randomized trials.
Conclusions
In this secondary analysis of AcT trial examining the safety and efficacy of tenecteplase versus alteplase in stroke patients with MeVO, we found similar clinical and safety outcomes, however, final successful reperfusion was better in patients receiving tenecteplase prior to EVT.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.03713.
Definition of MeVO arterial segments
Baseline characteristics of patients with medium vessel occlusions and treated with endovascular thrombectomy
Primary and secondary outcomes stratified by occlusion location and thrombolysis treatment type
Distribution of occlusion type in published tenecteplase trials
Distribution of scores in the EQ-5D-5L mobility domain. EQ-5D-5L, EuroQol Group 5-Dimension Self-Report Questionnaire.
Distribution of scores in the EQ-5D-5L self-care domain. EQ-5D-5L, EuroQol Group 5-Dimension Self-Report Questionnaire.
Distribution of scores in the EQ-5D-5L usual activities domain. EQ-5D-5L, EuroQol Group 5-Dimension Self-Report Questionnaire.
Distribution of scores in the EQ-5D-5L pain/discomfort domain. EQ-5D-5L, EuroQol Group 5-Dimension Self-Report Questionnaire.
Distribution of scores in the EQ-5D-5L anxiety/ depression domain. EQ-5D-5L, EuroQol Group 5-Dimension Self-Report Questionnaire.
Forest plot of adjusted risk ratio by treatment arm for the clinical and angiographic outcomes stratified by vessel occlusion site. Clinical outcomes (mRS 0–1 and mRS 0–2) were adjusted for age, sex, baseline stroke severity, stroke symptom onset-to-needle time as fixed-effects variables, and site as a random-effects variable. Angiographic outcomes were adjusted for age as fixed-effects variable, and site as a random-effects variable. P values for interaction were not significant for all outcomes (P>0.05). Adjusted risk ratio >1 is in favor of tenecteplase. For angiographic outcomes: occlusions other than M2-MCA were combined in one category because of the small numbers. mRS, modified Rankin Scale; MCA, middle cerebral artery; rAOL, revised arterial occlusion lesion score; eTICI, expanded Thrombolysis in Cerebral Infarction Score; CI, confidence interval.
Notes
Funding statement
This research was supported by Canadian Institutes of Health Research.
Conflicts of interest
Bijoy Menon has stock options in Circle NVI and has consulted for Biogen, Roche and Boehringer Ingelheim; Shelagh Coutts: Boehringer Ingelheim provides study drug (Tenecteplase) for the TEMPO-2 trial that Dr Coutts is the PI. Luciana Catanese received payments by Servier Inc., consulting fees from Ischaemavie RAPID, Circle NV and CMPA, Jai Jai Shankar has a grant from Medtronic to the University of Manitoba, Michael Hill has received consulting fees from Sun pharma, Brainsgate Inc, has stock options in Circle NVI, Tolu Sajobi has received consulting fees from Circle NVI, Rick Swartz has stock options in Follow-MD Inc, gets salary support for research from Heart & Stroke Foundation of Canada, Sandra Black Centre for Brain Resilience & Recovery and Ontario Brain Institute. The other authors have no disclosures.
Author contribution
Conceptualization: FBa, MA, BKM. Study design: FBa, MA, BKM. Methodology: FBa, NS, AA, TS, MA, BKM. Data collection: FBa, NS, IA, AA, FBe, MH, TS, MA, BKM. Statistical analysis: FBa, AA, TS, MA, BKM. Writing—original draft: FBa, MA, BKM. Writing—review & editing: all authors. Funding acquisition: AD, BB, MH, RS, MA, BKM. Approval of final manuscript: all authors.
Acknowledgements
We would like to thank Asmaa, Salma, and Ola Bala for their assistance in this research.