Association Between Anemia and Clinical Outcome in Acute Ischemic Stroke Patients Treated With Endovascular Treatment
Article information
Abstract
Background and Purpose
Endovascular treatment (EVT) is the preferred treatment option in eligible acute ischemic stroke (AIS) patients with a large vessel occlusion of the anterior circulation. Several comorbidities have been identified that can affect clinical outcomes. Various studies have investigated the association between anemia and clinical outcome and found conflicting results. The aim is to investigate the association between pre-EVT anemia and clinical outcomes at different time points post-EVT, primarily focusing on the National Institutes of Health Stroke Scale (NIHSS) at 24–48 hours.
Methods
We prospectively included 560 AIS patients who received EVT in the Maastricht University Medical Center+. Hemoglobin levels (Hb; g/dL) were determined on admission. Hb levels were also categorized into two groups: anemia (male: Hb ≤12.9 g/dL; female: Hb ≤11.9 g/dL) and no anemia. Multiple imputation was used to handle missing data. Multivariable regression was used to investigate the association between anemia or Hb levels and clinical outcomes.
Results
Anemia was present in 26% of the patients. Multivariable regression did not show a significant association between anemia or Hb levels and NIHSS at 24–48 hours (adjusted β [aβ]anemia: 1.44, 95% confidence interval [CI]: -0.47 to 3.36; aβHb: -0.37, 95% CI: -0.88 to 0.13). However, multivariable regression showed significant associations with modified Rankin Scale (adjusted common odds ratio [acOR]anemia: 1.66, 95% CI: 1.12 to 2.48; acORHb: 0.83, 95% CI: 0.75 to 0.93) and poor functional outcome at 90 days (adjusted OR [aOR]anemia: 2.09, 95% CI: 1.21 to 3.63; aORHb: 0.80, 95% CI: 0.69 to 0.92).
Conclusion
Anemia was not independently associated with early neurological deficit (NIHSS) post-AIS, suggesting it is more suitable as a general frailty marker.
Introduction
Several studies have shown that acute ischemic stroke (AIS) patients with a large vessel occlusion (LVO) of the anterior circulation benefit from endovascular treatment (EVT) [1-5]. Based on these results, EVT has become the standard of care in this patient population [6].
The clinical outcome of EVT can be affected by various comorbidities in the patients [7,8]. As such, previous studies suggested that anemia, predominantly caused by iron deficiency, is associated with increased morbidity and mortality in AIS patients, especially in cases of LVO [8-10].
Several studies found anemia on admission to be an independent predictor for poor clinical outcome post-AIS when treated with intravenous thrombolysis, EVT, or a combination of both [11-20]. Conversely, other studies did not find this association [10,21-23]. Although a large meta-analysis concluded a positive association between anemia on admission and adverse clinical outcomes, heterogeneity between the included studies was substantial [9]. Notably, while previous studies used the modified Rankin Scale (mRS) at 30 days to 1-year post-AIS as a primary outcome measure, an association between anemia and earlier outcome measures has not yet been assessed. It has been suggested that early outcome assessment, e.g., by means of the National Institutes of Health Stroke Scale (NIHSS) during hospitalization, would be more appropriate, as it may be less influenced by other clinical factors compared to the mRS and is more useful in practice [24-26]. As up to 40% of AIS patients suffer from anemia, it is relevant to further study the relationship between anemia and clinical outcomes post-EVT.
Therefore, the aim of this retrospective study was to investigate the association between pre-EVT anemia and clinical outcomes at different time points post-EVT, with a primary focus on the NIHSS at 24–48 hours.
Methods
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval for this study was obtained from the ethics committee of the Maastricht University Medical Center, Maastricht, The Netherlands (MEC-2020-1456). The need for individual patient consent was waived owing to the retrospective nature of the study.
Design and study population
This retrospective study used data from the Maastricht University Medical Center+ (MUMC+). Patients who received EVT between 2010 and 2019 were screened. Patients with an occlusion of the internal carotid artery (ICA), ICA-terminus, or middle cerebral artery segment M1 or M2 were included. The exclusion criteria were as follows: (1) patients randomized into the MR CLEAN MED trial who received trial medication (acetylsalicylic acid and/or unfractionated heparin) [27], as this could affect the clinical outcome; and (2) patients who were included in the MR CLEAN LATE trial, as researchers were still blinded for endpoint data during the analysis of the current study.
Study parameters
Clinical and procedural parameters were collected from prospective stroke records. Because a large part of the included patient cohort participated in the MR CLEAN trial, MR CLEAN Registry, MR CLEAN NO-IV, or MR CLEAN MED, core lab imaging assessments were often readily available [1,27-29]. If imaging parameters were missing, an experienced neuroradiologist and core lab member (A.P.) scored the respective parameters.
Hemoglobin levels (Hb; in g/dL) determined on admission were not included in the prospective stroke records and were retrospectively collected from patient medical files. Hb levels were assessed upon arrival at the emergency department, either in the referring center or our center (treating center), before the onset of the EVT. If the Hb level was not determined on admission at the emergency department, Hb levels assessed in the prior 7 days were used. Notably, in the case of major bleeding, Hb levels were deemed missing and were therefore imputed. When multiple Hb levels were available, the level obtained closest to the EVT was used for analyses in the present study. Anemia was defined according to the World Health Organization criteria (Hbmale ≤12.9 g/dL; Hbfemale ≤11.9 g/dL) [30,31].
The primary outcome was the NIHSS score at 24–48 hours post-EVT. Secondary outcomes were the mRS score, poor functional outcome (mRS 3–6), and mortality at 90 days post-EVT. The NIHSS and mRS scores were prospectively assessed by trained personnel. Specifically, the mRS was assessed during a phone interview at 90 days post-AIS, which is the standard follow-up procedure for all stroke patients in the Netherlands. Missing follow-up NIHSS scores were, if possible, retrospectively reconstructed based on the patient’s medical file by a trained researcher (F.P.). The mRS scores were not retrospectively reconstructed. Hence, missing mRS scores were always imputed.
Missing data
In the included cohort, Hb levels were missing in 13% (73/560) of the patients. Considering our outcome measures, 5.7% of NIHSS scores at 24–48 hours and 4.1% of mRS scores at 90 days post-EVT were missing. The missing data percentage in all other baseline variables included in the analyses was 4.6%.
Multiple imputation by chained equations (MICE) using the mice package version 3.15.0 (https://cran.r-project.org/) was used to handle the missing data [32]. The imputation model included relevant covariates and outcome variables. The number of imputations was based on the fraction of missing information [33].
Statistical analysis
Owing to this study’s explorative and retrospective nature, a power calculation was not performed.
Statistical analysis was performed with R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). Crude data were used to describe baseline patient characteristics, laboratory parameters, and imaging and EVT characteristics of patients with and without anemia. In addition, a baseline table based on the presence/absence of Hb was provided to evaluate for potential imbalances. To test for significant differences between the anemia and no-anemia groups, the chi-square, Fisher’s exact, Student’s t, and Mann-Whitney U tests were used as appropriate.
Continuous data are presented as means±standard deviation or medians with interquartile range (IQR), based on the nature of the data. NIHSS scores were analyzed as a continuous variable.
Uni- and multivariable linear, binary logistic, and ordinal regression were used as appropriate to determine the association between Hb levels or anemia and outcome variables. Variables with P<0.10 in univariable regression analyses were included in the multivariable regression. To prevent overfitting of the regression models, the maximum number of confounders was restricted to 10% of the outcomes per outcome variable [34]. The effect estimates of linear regression are presented per 1 g/dL decrease in Hb.
Results
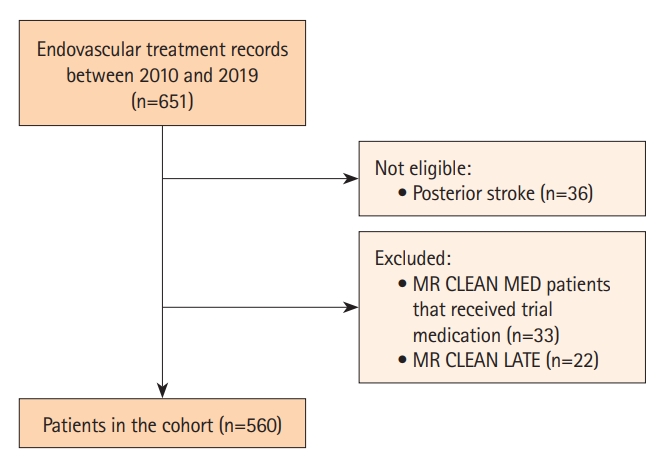
In total, 651 EVT records were screened for eligibility, of which 560 patients were included for statistical analysis. An inclusion flowchart is presented in Figure 1. Baseline characteristics are described in Table 1. Anemia was present in 26% of the patients. AIS patients suffering from anemia were slightly older with a median age of 77 years, compared to 70 years in non-anemic AIS patients (P<0.001). In addition, anemia was more common in female patient (56%) than in male patients, although not statistically significant (P=0.469). Median Hb levels of anemic patients were 11.3 g/dL and 12.1 g/dL for female and male anemic patients, respectively. A medical history of cardiovascular risk factors was more frequently seen in the anemia group (P=0.011). Consequently, patients suffering from anemia were more frequently on antihypertensive (P<0.001), cholesterol-lowering (P=0.048), antiplatelet (P=0.007), or anticoagulation medication (P=0.011). In addition, as shown in Supplementary Table 1, patients with a known versus an unknown Hb level were comparable in terms of stroke severity on admission and pre-morbid mRS, with only minimal differences in stroke history, the number of M1 occlusions, and total EVT-attempts.
Association with outcome measures
Effect estimates of the performed regression analyses are presented in Table 2. In the univariable analysis, both anemia and decreased Hb levels were significantly associated with an increase in NIHSS at 24–48 hours post-EVT (βanemia: 2.87, 95% confidence interval [CI]: 0.60 to 5.14; βHb: -0.80, 95% CI: -1.37 to -0.23). However, this was no longer significant in the multivariable regression analysis (adjusted [a]βanemia: 1.44, 95% CI: -0.47 to 3.36; and aβHb: -0.37, 95% CI:-0.88 to 0.13).
Considering the mRS at 90 days, both anemia and decreasing Hb levels were significantly associated with an mRS shift to poorer outcomes in the univariable regression analysis (common odds ratio [cOR]anemia: 2.01, 95% CI: 1.45 to 3.04; and cORHb: 0.78, 95% CI: 0.71 to 0.86). This effect remained significant in the multivariable regression analysis (adjusted cOR [acOR]anemia: 1.66, 95% CI: 1.12 to 2.48; and acORHb: 0.83, 95% CI: 0.75 to 0.93). In addition, relevant interaction terms were explored and, if significant, are reported in the supplemental materials (Supplementary Tables 2-9). As such, the interaction term of Hb*age is positively associated with mRS at 90 days (acORHb*age: 0.995, 95% CI: 0.991–0.999) (Supplementary Table 5).
Likewise, there was a significant association with a poor functional outcome in the univariable analysis (ORanemia: 2.49, 95% CI: 1.56 to 3.97; ORHb: 0.76, 95% CI: 0.68 to 0.86). This effect remained significant in the multivariable regression analysis (aORanemia: 2.09, 95% CI: 1.21 to 3.63; aORHb: 0.80, 95% CI: 0.69 to 0.92).
Anemia and a decrease in Hb levels were significantly associated with an increase in mortality at 90 days in the univariable regression analysis (ORanemia: 2.30, 95% CI: 1.48 to 3.58; ORHb: 0.77, 95% CI: 0.69 to 0.87). However, this effect was no longer significant in the multivariable regression analysis (aORanemia: 1.53, 95% CI: 0.88 to 2.66; aORHb: 0.86, 95% CI: 0.74 to 1.01).
Lastly, a sensitivity analysis with unimputed hemoglobin levels (i.e., a dataset containing 487/560 patients) is presented in Supplementary Table 10 and showed similar results as compared to the analyses with the imputed dataset.
Discussion
In this study, we aimed to identify an association between preEVT anemia and clinical outcomes in AIS patients with an LVO of the anterior circulation. Our main findings were that, in multivariable regression analysis, both anemia and Hb levels were significantly associated with the mRS and poor functional outcome at 90 days, but not with the NIHSS at 24–48 hours or mortality at 90 days.
Unlike other studies with a similar research question, we used the NIHSS at 24–48 hours as our primary outcome measure. As the presence of anemia is often accompanied by other comorbidities [35-39], the true effect of anemia on clinical outcome may be masked when using only long-term outcome measures [24,25]. Additionally, non-specific functional outcome measures (i.e., mRS or the Barthel Index), which are assessed after a typical recovery period of 90 days, might not accurately represent the relationship between anemia and neurological deficit immediately after AIS [24,25]. However, anemia and decreased Hb levels were not significantly associated with the NIHSS at 24–48 hours in our multivariable analyses, which may suggest that anemia is more suitable as a general frailty marker rather than as a marker for neurological deficit immediately post-AIS. This hypothesis is supported by the finding that the presence of anemia and lower Hb levels were significantly associated with advanced age and the presence of comorbidities in the present study. Additionally, Supplementary Table 5 shows that there is a significant effect of age on the association between Hb and mRS at 90 days. Noteworthy, the cOR of the interaction was 0.995, indicating that this effect decreased with increasing age, presumably due to the inverse relationship between age and Hb level. Nevertheless, this effect is minimal and has questionable clinical relevance and is therefore only shown in the supplemental materials (Supplementary Table 5). However, given the retrospective nature of the present study, we did not evaluate frailty in a specific way. Therefore, this hypothesis has to be interpreted with caution.
Previous literature has suggested that anemic patients, who likely also suffer from additional comorbidities, already have impaired cerebral autoregulation before stroke onset [40]. Additionally, a previous study proposes that during stroke, the cerebral oxygen uptake in the penumbral region progressively decreases with Hb levels below 10.0 g/dL. Consequently, one could reason that it might be more difficult to tolerate hypoxic/ischemic events in the presence of anemia with additional comorbidities compared to AIS patients without anemia and other comorbidities [10].
Besides the NIHSS at 24–48 hours, we investigated the association between anemia or lower Hb levels and poor functional outcome or mortality at 90 days. We found a significant association between the presence of anemia or decreased Hb levels and poor functional outcome, but not with mortality. These results are in line with previous studies [14,15]. Of note, one study only found this association in patients with moderate (Hb <10.0 g/dL for both sexes) to severe anemia (Hb <8.0 g/dL for both sexes)[ 10]. Our study population included only few patients suffering from moderate to severe anemia, rendering subgroups too small for statistical analysis with adequate power. Notably, approximately 55% of the patients included in the previously mentioned cohort suffered from mild anemia, while this proportion was roughly 67% in our cohort. Interestingly, since this previously mentioned study only included 90 anemic patients in total, it could be possible that there was not enough power to detect an association with mild anemia. Markedly, during the clinical follow-up, the aforementioned study performed restrictive red blood cell transfusion (RBCT), i.e., RBCT in patients with Hb levels <8.0 g/dL. Our center does not routinely provide RBCT in this patient population as the optimal RBCT strategy and threshold in AIS patients is still under debate [41,42].
While previous studies demonstrated a significant association between anemia and mortality at 90 days, our present study found no such effect [12,14,15]. Possible explanations for this unexpected result could be differences between study populations, i.e., mean population age, baseline NIHSS score, and correction for different confounders.
Several limitations have to be addressed. Firstly, the small sample sizes in the moderate to severe anemia subgroups did not allow for subgroup analysis on this subject. Secondly, although this study used prospectively collected data, Hb levels were collected retrospectively. In addition, it was not possible to determine the etiology (e.g., nutritional status) of anemia/low Hb. Consequently, we did not distinguish between different types of anemia (e.g., iron deficient anemia, vitamin deficient anemia, and hemolytic anemia) and had no information regarding the underlying cause of anemia, which can affect functional dependence and mortality [36]. In that line, anemic patients had significantly higher C-reactive protein levels than that in non-anemic patients (Table 1). Unfortunately, owing to the retrospective nature of the present study, we were unable to collect information regarding comorbidities other than cardiovascular risk factors and corresponding medication. Thirdly, though we had a very extensive clinical dataset it is possible that we did not adjust for all relevant confounders (e.g., computed tomography perfusion parameters).
Despite these limitations, there are several strengths of this study. First, our dataset was larger compared to that in other studies [8,14,23]. Second, while the majority of the studies on this topic use the mRS and/or mortality as a primary outcome measure, we used a neurological deficit score (NIHSS) early post-EVT as a primary outcome measure as it may be less influenced by other clinical factors [24,25]. Lastly, we used a multiple imputation strategy to handle missing data in both the dependent and independent variables. In addition, we performed a sensitivity analysis with unimputed hemoglobin data (i.e., data from 487 patients of whom a hemoglobin level was available upon hospital admission) and found no discrepancies as compared with the imputed data analysis, showing no biases introduced by the imputation strategy (Supplementary Table 10).
Conclusions
Anemia was found to be significantly associated with clinical outcomes at 90 days post-EVT in patients with an LVO of the anterior circulation. Given the fact we did not observe an association with the NIHSS at 24–28 hours post-EVT, it seems more likely that anemia is a marker for frailty rather than an early neurological outcome marker after AIS.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.01669.
Baseline patient characteristics and laboratory parameters based on the presence of hemoglobin levels (n=560)
Model output of linear regression analysis for the association between anemia and NIHSS at 24-48 hours
Model output of linear regression analysis for the association between hemoglobin level on admission and NIHSS at 24-48 hours
Model output of ordinal logistic regression analysis for the association between anemia and mRS at 90 days
Model output of ordinal logistic regression analysis for the association between hemoglobin level on admission and mRS at 90 days
Model output of binary logistic regression analysis for the association between anemia and mRS 3–6 at 90 days
Model output of binary logistic regression analysis for the association between hemoglobin level on admission and mRS 3–6 at 90 days
Model output of binary logistic regression analysis for the association between anemia and mortality at 90 days
Model output of binary logistic regression analysis for the association between hemoglobin level on admission and mortality at 90 days
Sensitivity analyses without imputed hemoglobin values
Notes
Funding statement
None
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: RJvO, AC, FMEP. Study design: AC, FMEP. Methodology: AC, FMEP. Data collection: AC, FMEP. Investigation: AC, FMEP. Statistical analysis: AC. Writing—original draft: AC. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Acknowledgements
We thank the MR CLEAN trial, MR CLEAN Registry, MR CLEAN NO-IV, and MR CLEAN MED for providing part of the data.
Anonymized data from this study can be made available to other researchers, upon reasonable request to the corresponding author.