Endovascular Thrombectomy for Large Ischemic Strokes: A Living Systematic Review and Meta-Analysis of Randomized Trials
Article information
Abstract
Background and Purpose
New studies have shown that endovascular thrombectomy (EVT) is safe and effective for acute ischemic stroke (AIS) patients with large ischemic areas. The aim of our study is to conduct a living systematic review and meta-analysis of randomized trials comparing EVT versus medical management only.
Methods
We searched MEDLINE, Embase, and the Cochrane Library to identify randomized controlled trials (RCTs) comparing EVT versus medical management alone in AIS patients with large ischemic regions. We conducted our meta-analysis using fixed-effect models to compare functional independence, mortality, and symptomatic intracranial hemorrhage (sICH) between EVT and standard medical management only. We assessed the risk of bias using the Cochrane risk-of-bias tool and the certainty of evidence for each outcome using the Grading of Recommendations, Assessment, Development, and Evaluations approach.
Results
Of 14,513 citations, we included 3 RCTs with a total of 1,010 participants. We found low-certainty evidence of possibly a large increase in the proportion of patients with functional independence (risk difference [RD] 30.3%, 95% CI 15.0% to 52.3%), low-certainty evidence of possibly a small non-significant decrease in mortality (RD -0.7%, 95% CI -3.8% to 3.5%), and low-certainty evidence of possibly a small non-significant increase in sICH (RD 3.1%, 95% CI -0.3% to 9.8%) for AIS patients with large infarcts who underwent EVT compared to medical management only.
Conclusion
Low-certainty evidence shows that there is possibly a large increase in functional independence, a small non-significant decrease in mortality, and a small non-significant increase in sICH amongst AIS patients with large infarcts undergoing EVT compared to medical management only.
Introduction
Endovascular thrombectomy (EVT) is now the gold standard treatment modality for acute ischemic stroke (AIS) caused by large vessel occlusion (LVO) [1]. The current guidelines for AIS management state that only patients with an Alberta Stroke Program Early Computed Tomography Score (ASPECTS) ≥6 are eligible for EVT [1,2]. Although AIS patients with large ischemic region on neuroimaging represent a considerable proportion of LVO strokes (around 20%), those patients were excluded or underrepresented in EVT trials due to the concern of bleeding from reperfusion [3-6]. However, 3 randomized clinical trials (RCT) were recently conducted to assess the efficacy of EVT versus standard medical care alone in AIS patients with large-sized ischemic regions in the initial neuroimaging studies [7-9]. Therefore, we conducted this timely living systematic review and meta-analysis to provide robust evidence on the efficacy and safety of EVT in AIS patients with large ischemic region.
Methods
Standardized reporting and registration
We adhered to Cochrane systematic review methodology and formatted the review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline (Supplementary Table 1) [10,11]. We prospectively registered the systematic review protocol on the International Prospective Register of Systematic Reviews (PROSPERO, CRD42023398742).
Data sources and searches
With the aid of an information specialist, we searched MEDLINE (through Ovid), Embase, and the Cochrane Library from inception to February 10, 2023. We did not use any language restrictions. The detailed search strategies are described in Supplementary Table 2. We also hand-searched the grey literature and the reference lists of included studies.
Study selection
We included records addressing the following eligibility criteria:
Population
We sought studies including adult patients aged 18 years or older presenting with AIS and are found to have a large ischemic area on baseline neuroimaging, defined as having an ASPECTS value of less than 6 regardless of imaging modality used or having an infarct core volume of at least 50 mL. We planned to evaluate different subgroups, including different core infarct sizes and imaging criteria. We excluded in vitro, animal, or post-mortem studies.
Intervention
We sought studies comparing EVT to standard medical management, regardless of time from onset to treatment. We also determined a priori to evaluate different types of endovascular procedures performed adjunctively to thrombectomy (e.g., stenting, angioplasty).
Outcomes
Outcomes of interest were the proportion of patients who achieved functional independence (defined as a modified Rankin Scale [mRS] score of 0 to 2, at 90 days); mortality as death at 90 days after stroke onset due to any cause; symptomatic intracranial hemorrhage (sICH) (defined as any intracranial hemorrhage associated with worsening neurologic exam, clinical deterioration, or death, adapted from the Heidelberg Bleeding Classification) [12]; proportion of patients with an mRS of 0 to 3 at 90 days; improvement in any domain on the National Institutes of Health Stroke Scale (NIHSS) at 24 to 48 hours after randomization; and any intracranial hemorrhage.
Study designs
We included RCTs only given their high quality of evidence and for quantitative assessment. We excluded all other study designs, such as observational studies, editorials, commentaries, guidelines, literature reviews, systematic reviews and meta-analyses, conference abstracts, and news articles.
Screening
Reviewers participated in calibration exercises using piloted standardized screening forms prior to the screening process. Teams of two reviewers independently screened and verified each citation. Subsequently, we retrieved the full texts of those citations that were deemed potentially eligible. Each full text was screened by one reviewer and another reviewer verified its eligibility. A third reviewer resolved any disagreements when necessary.
Data collection
Reviewers extracted data from each eligible RCT independently into an Excel spreadsheet via Microsoft Excel 2021 and verified the extracted data in duplicate using previously developed standardized data abstraction forms. Reviewers extracted the following characteristics: study characteristics (e.g., country, study design), patient characteristics (e.g., sample size, age, sex, prior medical history, NIHSS score, vessel involved, tandem occlusion, stroke etiology), intervention and comparator details (e.g., intravenous thrombolytic agent), and management outcomes, including mRS scores, mortality, intracranial hemorrhage, and procedural complications.
Risk of bias assessment
Two reviewers independently assessed the quality of included trials in strict accordance using the revised Cochrane risk-of-bias tool (RoB 2) for randomized controlled trials [13]. We improved the reliability of RoB 2, especially low interrater reliability and complex terminology, by training the authors prior to implementation [14]. Any discrepancies between the two assessors were resolved through discussion and including a third assessor.
Data synthesis and analysis
We synthesized the data in narrative and tabular formats. We conducted meta-analysis using the Mantel-Haenszel method to calculate risk ratios (RRs) and the associated 95% confidence intervals (CIs), for all patient-important outcomes reported by more than one study. We used the crude event rate and the associated CIs to calculate the RRs for dichotomous outcomes. For computing risk differences (RDs) and 95% CIs, we applied the RRs to the baseline risks from a well-designed, high-quality multi-center prospective cohort study of 2,420 patients with acute large vessel occlusions [15]. When median and range values were reported, or when standard deviations (SDs) were not reported, we estimated the means and standard deviations used methods detailed in the Cochrane Handbook [10] and by Wan et al. [16]. We synthesized our findings into funnel plots to assess for asymmetry for each outcome, and evaluated for statistical heterogeneity using inconsistency measures, Cochran’s Q test and I2. We planned to perform subgroup analysis if there were at least two studies reporting outcomes per subgroup, irrespective of heterogeneity. All statistical analyses were done by Stata/MP version 17 for Microsoft Windows (StataCorp, College Station, TX, USA).
Certainty of evidence assessment
We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach to assess the certainty of evidence for each outcome (high, moderate, low, or very low), and developed GRADE evidence profiles using the GRADEpro app (www.gradepro.org) [17,18]. We used the following standard terminology to describe the strength of comparison for each outcome: “there is” for high-certainty of evidence, “there probably is” for moderate-certainty of evidence, and “there possibly is” for low- or very low-certainty of evidence. We used previously derived minimally important different thresholds for functional independence, mortality, and sICH from a previously developed guideline panel strategy [19].
Living review and literature surveillance
We will use our search strategies to perform monthly updates or alerts. If new evidence is available that is sufficient to change the overall assessment, certainty of evidence, and provide more information on additional outcomes, then we would meta-analyze these findings and report the results in an updated manuscript.
Results
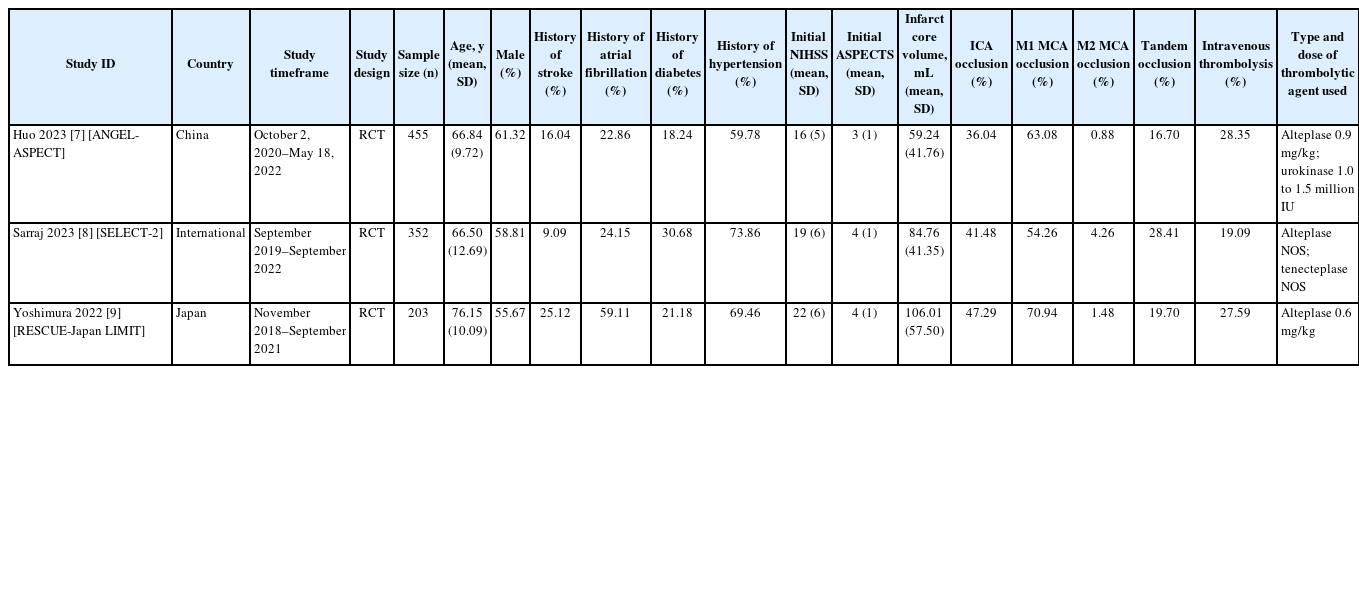
Of 14,513 citations, we identified 16 potentially eligible studies and included 3 RCTs with a total of 1,010 participants (Figure 1). The summary of the 3 RCTs is found in the Graphical Overview for Evidence Reviews (GOfER) diagram (available at https://ibb.co/26FWhyK).
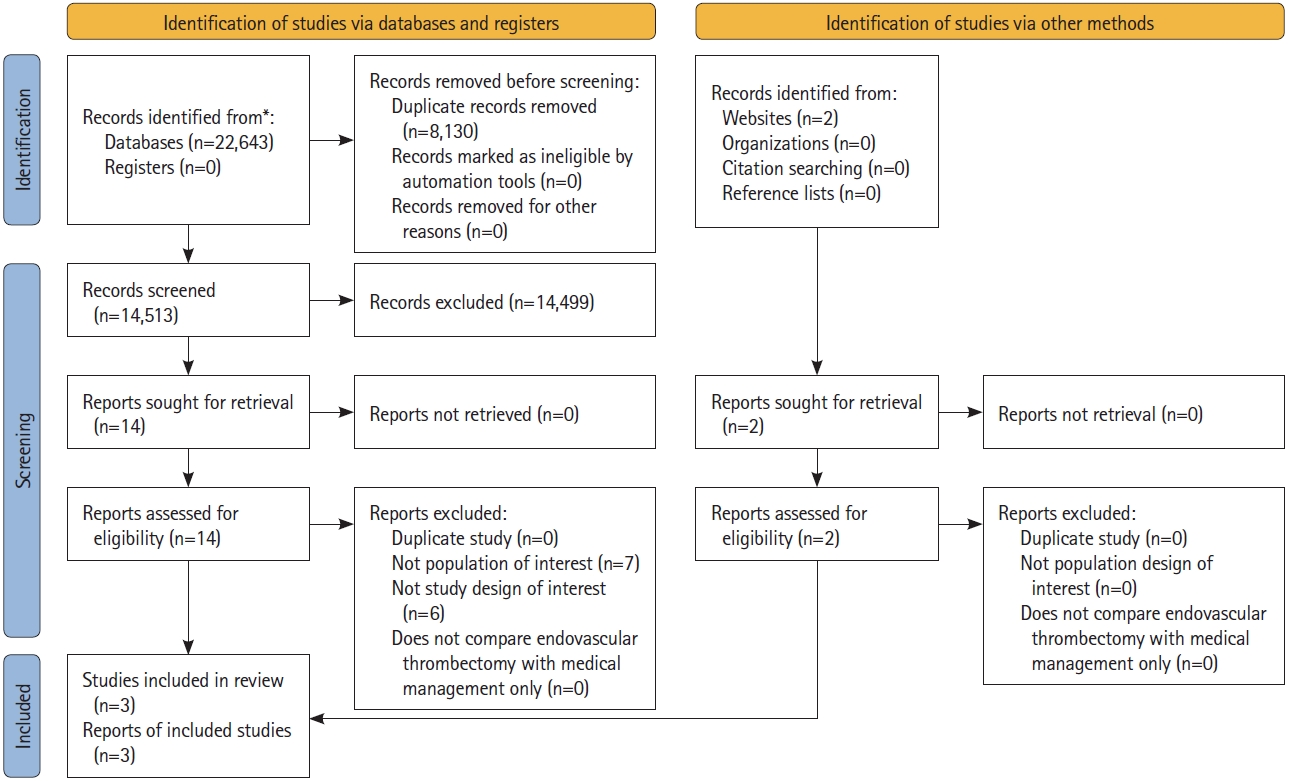
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. *Consider, if feasible to do so, reporting the number of records identified from database or register searched (rather than the total number across all databases/registers). Adapted from Page et al. J Clin Epidemiol 2021;134:178-189, under the Creative Commons license (CC-BY).[11] For more information, visit http://www.prisma-statement.org.
Characteristics of included studies
Table 1 describes the characteristics of the included RCTs reporting on outcomes for AIS patients with large ischemic regions who underwent EVT versus those who received medical management only [7-9]. The number of included patients ranged from 203 to 455 [7-9], two studies used alteplase at doses ranging from 0.6 to 0.9 mg/kg [7,9], and one study used alteplase or tenecteplase at unspecified doses [8]. Moreover, one study also used urokinase [7]. The average ASPECTS at baseline ranged from 3 to 4, and the core infarct volume ranged from 59.24 mL to 106.01 mL [7-9]. The rate of procedural complications, including arterial dissections, perforations, and arterial access site complications, was 12.2% (n= 62/509) among the EVT group from all studies [7-9].
Risk of bias assessment
All RCTs used adequate randomization generation methods, reported less than 10% missing outcome data, and blinded outcome assessment; however, participants or providers were not blinded given the need for EVT. Most RCTs were rated as low risk of bias but one RCT was rated as high risk of bias due to inadequate randomization methods. Additionally, some cointerventions were introduced that may have affected study outcomes given the inability to blind participants or providers. Details pertaining to the risk of bias assessment are highlighted in Supplementary Figure 1.
Outcomes for EVT versus medical management only
Functional independence (mRS score 0–2 at 90 days)
All studies reported on functional independence, defined as an mRS score of 0 to 2 at 90 days [7-9]. We found low-certainty evidence suggesting that there is possibly a large increase in the proportion of patients achieving functional independence in the EVT group in comparison to those with medical management only (RR 2.53, 95% CI 1.76 to 3.64; RD 30.3%, 95% CI 15.0% to 52.3%) (Table 2 and Figure 2).
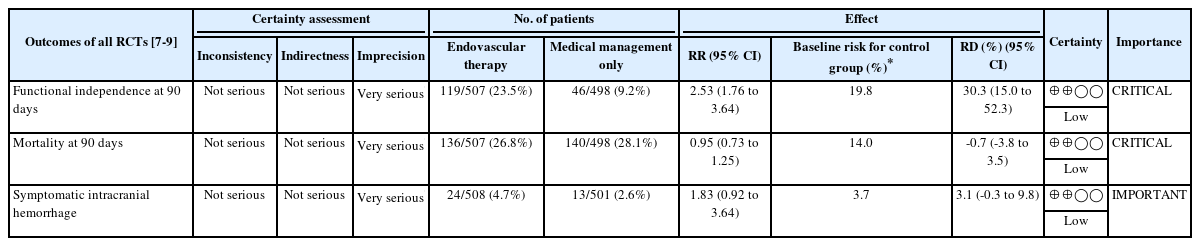
GRADE evidence profile for all RCTs comparing outcomes for acute ischemic stroke patients who underwent EVT versus medical management only
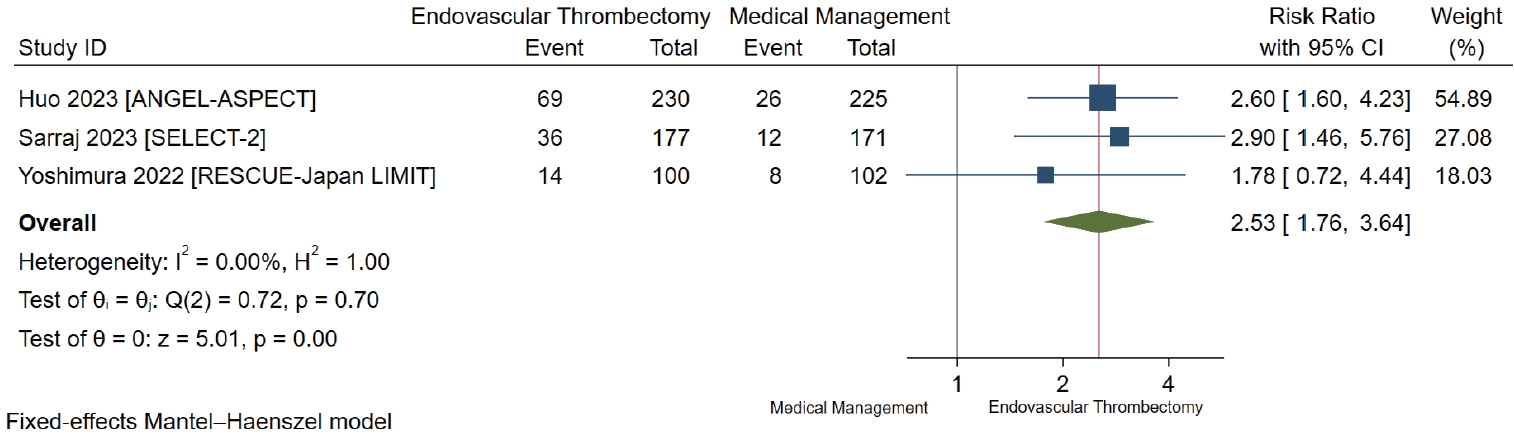
Forest plot for endovascular thrombectomy versus medical management only for functional independence defined as modified Rankin Scale score of 0 to 2 at 90 days. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; SELECT- 2, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial; CI, confidence interval.
Mortality at 90 days
All studies reported mortality at 90 days [7-9]. We found low-certainty evidence suggesting that there is possibly a small nonsignificant decrease in mortality at 90 days for patients who underwent EVT compared to those with standard medical management (RR 0.95, 95% CI 0.73 to 1.25; RD -0.7%, 95% CI -3.8% to 3.5%) (Table 2 and Figure 3).
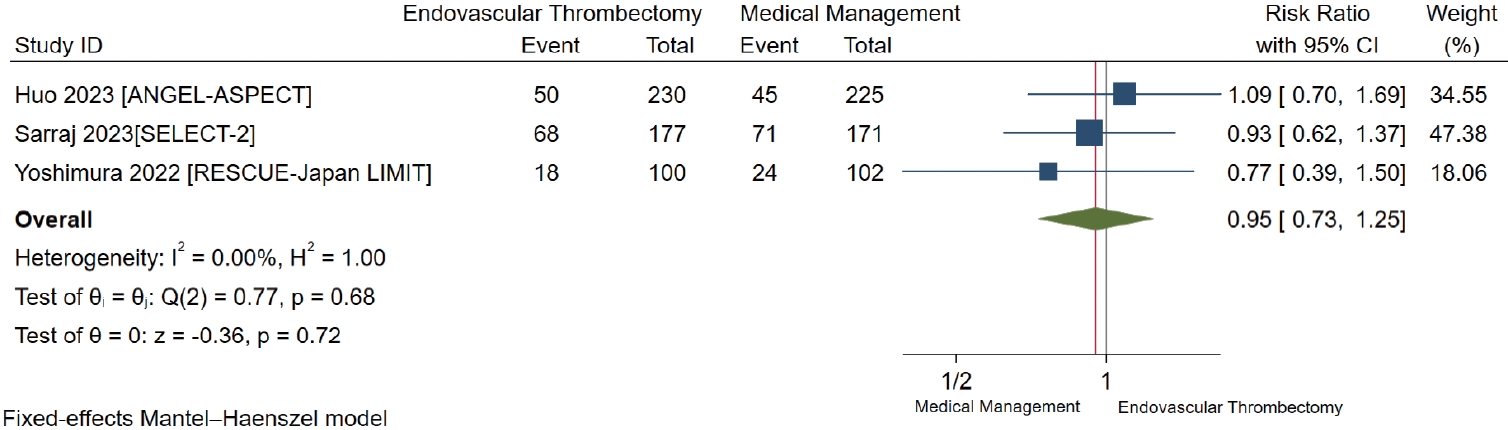
Forest plot for endovascular thrombectomy versus medical management only for mortality at 90 days. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; SELECT-2, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial; CI, confidence interval.
Symptomatic intracranial hemorrhage
All studies reported sICH with differences in the definitions used between studies [7-9]. We found low-certainty evidence suggesting that there is possibly a small non-significant increase in sICH, at least 24 to 48 hours after randomization, in patients who underwent EVT compared to those with medical management only (RR 1.83, 95% CI 0.92 to 3.64; RD 3.1%, 95% CI -0.3% to 9.8%) (Table 2 and Figure 4).
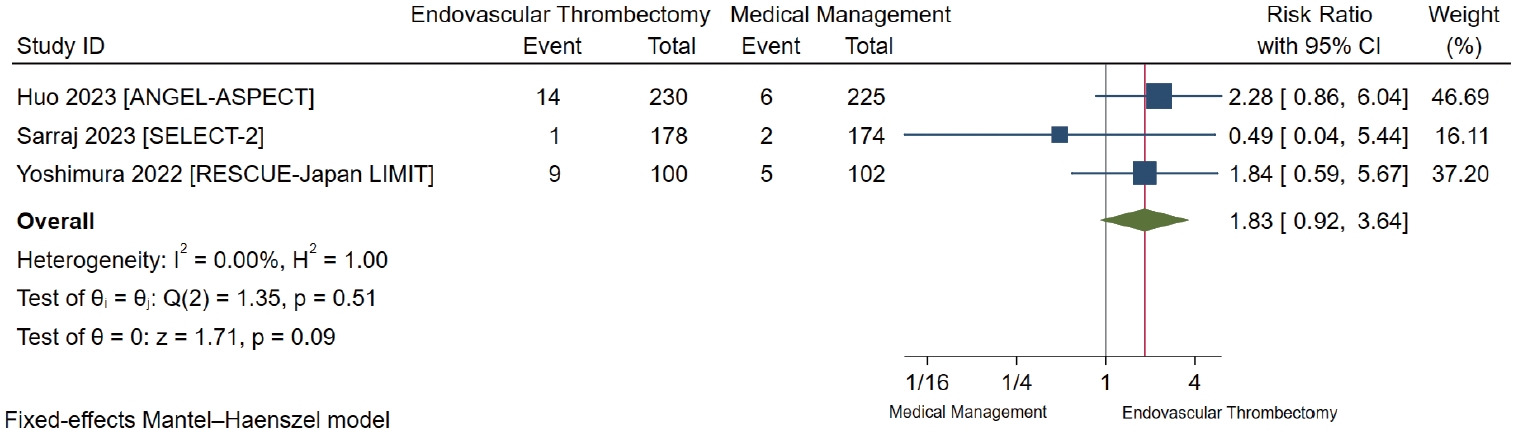
Forest plot for endovascular thrombectomy versus medical management only for symptomatic intracranial hemorrhage. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; SELECT-2, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultraacute Embolism Japan Large IscheMIc core Trial; CI, confidence interval.
Other secondary outcomes
All studies also reported on mRS of 0 to 3 at 90 days (RR 1.70, 95% CI 1.31 to 2.20) and improvement in the NIHSS score with variability in the definitions (RR 2.44, 95% CI 1.51 to 3.93) [7-9]. Only two studies reported on any intracranial hemorrhage (RR 2.41, 95% CI 1.76 to 3.32) [7,9]. Further details on these findings are illustrated in Supplementary Figure 2.
Discussion
This systematic review and meta-analysis aimed to evaluate EVT versus medical management alone in AIS patients with large infarct regions. We identified 3 RCTs meeting the inclusion criteria [7-9]. Our analysis produced low-certainty evidence suggesting that EVT possibly increases functional independence, represented by mRS score of 0 to 2, non-significantly decreases mortality, and non-significantly increases the risk of sICH. Imprecision and variability in outcome measurements between studies accounted for most of the quality of evidence assessment.
While there are a few systematic reviews evaluating EVT in AIS patients with large ischemic core volume [20-22], this meta-analysis is the first to incorporate evidence from the most recently published RCTs, namely the ANGEL-ASPECT (Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core), SELECT-2 (A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke), and RESCUE-Japan LIMIT (Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial) trials [7-9]. Moreover, we aim to make this study a living systematic review and meta-analysis to include future RCTs comparing outcomes in AIS patients with large infarct regions who underwent EVT versus medical management only. Our systematic review adhered to full living systematic review methodology, used rigorous methods in screening regardless of language, assessed for certainty of evidence using the GRADE approach, and evaluated the risk of bias using the RoB 2 tool. We engaged research methodologists and content experts to facilitate absolute risk quantification by imputing RDs for a more comprehensive interpretation of the results.
Although the results of this meta-analysis showed that EVT may be considered as an option in AIS patients with large ischemic core regions compared to standard medical management, there are certain distinctions that need to be made. RCTs were included regardless of the diagnostic modality used to identify large strokes. This distinction is important to make because bias can be introduced if studies using different diagnostic modalities other than baseline ASPECTS as an inclusion criterion are not included. While the baseline ASPECTS was considered when enrolling patients in the ANGEL-ASPECT, RESCUE-Japan LIMIT, and SELECT-2, the SELECT-2 trial used computed tomography perfusion or magnetic resonance imaging perfusion with any core volume greater than 50 mL in addition to ASPECTS to define large strokes [7-9]. Compared to other systematic reviews and meta-analyses, this study is focused more on patient-important outcomes rather than surrogate outcomes, such as any intracranial hemorrhage and successful reperfusion on final angiogram. There is a gap between surrogate endpoints and interpretation of these surrogate endpoints in clinical practice, and we focused on hard endpoints rather than surrogate endpoints to inform patient-centered care [19,23]. While we reported surrogate endpoints, such as any intracranial hemorrhage, we did not incorporate this data into our evidence assessment. Additionally, the included trials in this meta-analysis used different definitions of outcomes. The ANGEL-ASPECT trial defined sICH according to the Heidelberg Bleeding Classification [7], SELECT-2 used the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria [8], and the RESCUE-Japan LIMIT trial defined sICH as parenchymal hematoma type 2 encompassing at least 30% of the infarcted area in conjunction with worsening of the NIHSS score by at least 4 points within 48 hours [9]. Taking this variability of definitions into consideration, our pooled effect estimates did not show any statistical heterogeneity (I2=0%), thus making our conclusion such that EVT possibly increases the risk of sICH compared to medical management only. Finally, unlike prior systematic reviews, this is the first living systematic review on the topic given the anticipation for future trials addressing our question even further.
Factors that were not accounted for in this meta-analysis were the inability to perform a meta-regression analysis due to the number of studies and inadequate power, the inability to perform subgroup analyses based on certain characteristics, such as simultaneous presence of ipsilateral extracranial (or tandem) occlusions or type of intravenous thrombolytic agent used, and the inability to account for ordinal mRS shifts in our meta-analysis, which may serve as better measures for long-term endpoints compared to dichotomous endpoints [24].
Although our meta-analysis only included data from RCTs, it serves as a snapshot of the highest quality evidence available comparing EVT to standard medical management in AIS patients with large ischemic regions. It is important to understand that while these RCTs had differences in study design, patient inclusion criteria, imaging selection criteria, and thrombolytic agent type and dosing, outcomes were comparably similar. Additionally, two RCTs considered ordinal shift in mRS at 90 days as their primary outcome [7,8], and one RCT considered an mRS score of 0 to 3 at 90 days after stroke onset as the primary outcome [9]. Although EVT may be considered in death prevention, it may be associated with severe post-procedural functional deficits when factoring the benefits of EVT on the basis of an ordinal mRS shift [25]. Patients who underwent EVT had a shift towards improved outcomes in other categories based on the results of these trials, but mortality was still high ranging from 7.8% to 38.4% among the EVT group [7-9]. Our results also demonstrated low-certainty evidence suggesting a possible small increase in sICH among patients who underwent EVT compared to medical management only. While the SELECT-2 trial had the lowest risk of sICH compared to the other two included trials, patients who underwent EVT had higher rates of early neurologic worsening on exam, which could be speculated as post-procedural reperfusion-related edema [7-9]. This anticipated risk of sICH should be discussed with patients and caregivers and weighed against the possible increase in functional outcomes. It is also difficult to interpret the cost utility behind the use of EVT compared to standard medical management in this patient population, but one could extrapolate the greater costs for the required care of these patients. Of noteworthy importance, ANGEL-ASPECT did not enroll patients with ischemic core volumes larger than 70 mL to 100 mL with an ASPECTS greater than 5, and RESCUE-Japan LIMIT and SELECT-2 excluded AIS patients with ASPECTS less than 3; as such, outcomes and costs may vary if these subgroups are explored. Evidence from future trials, such as LASTE (Large Stroke Therapy Evaluation), TENSION (Efficacy and Safety of Thrombectomy in Stroke with Extended Lesion and Extended Time Window), and TESLA (Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke) [26], might help address the unanswered questions. Therefore, clinicians and guideline developers should cautiously interpret the results of this systematic review and meta-analysis when considering EVT for large strokes.
Conclusions
In conclusion, our living systematic review and meta-analysis examined the benefits and safety of EVT compared to medical management in AIS-LVO patients with large infarct regions. We found low-certainty evidence that EVT possibly increases functional independence, non-significantly decreases mortality, and non-significantly increases the risk of sICH. Evidence from anticipated future trials will further determine whether these outcomes remain valid.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.00752.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist
Search algorithms for MEDLINE (through Ovid), Embase, and Cochrane Library
Risk of bias assessment. (A) Revised Cochrane risk-of-bias tool (RoB2). (B) Risk of bias summary. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; SELECT-2, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial.
Other secondary outcomes. (A) Modified Rankin Scale score 0 to 3 at 90 days. (B) Improvement in National Institutes of Health Stroke Scale score. (C) Any intracranial hemorrhage. ANGEL-ASPECT, Study of Endovascular Therapy in Acute Anterior Circulation Large Vessel Occlusive Patients With a Large Infarct Core; SELECT-2, A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke; RESCUE-Japan LIMIT, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial; CI, confidence interval.
Notes
Funding statement
None
Conflicts of interest
The authors completed the ICMJE Disclosure Forms and declare no competing interests.
Author contribution
Conceptualization: RZM, ME, FA, TK. Study design: RZM, ME, HSG. Methodology: RZM, ME, HSG. Data collection: RZM, ME, HSG, MA, AE, SK, HD. Investigation: all authors. Statistical analysis: RZM, HSG. Writing—original draft: RZM, ME, HSG. Writing— review & editing: all authors. Approval of final manuscript: all authors.