Moderate-Intensity Rosuvastatin Plus Ezetimibe Versus High-Intensity Rosuvastatin for Target Low-Density Lipoprotein Cholesterol Goal Achievement in Patients With Recent Ischemic Stroke: A Randomized Controlled Trial
Article information
Abstract
Background and Purpose
Moderate-intensity statin plus ezetimibe versus high-intensity statin alone may provide a greater low-density lipoprotein cholesterol (LDL-C) reduction in patients with recent ischemic stroke.
Methods
This randomized, open-label, controlled trial assigned patients with recent ischemic stroke <90 days to rosuvastatin/ezetimibe 10/10 mg once daily (ROS10/EZT10) or to rosuvastatin 20 mg once daily (ROS20). The primary endpoint was LDL-C reduction ≥50% from baseline at 90 days. Key secondary endpoints were LDL-C <70 mg/dL and multiple lipid goal achievement, and composite of major vascular events.
Results
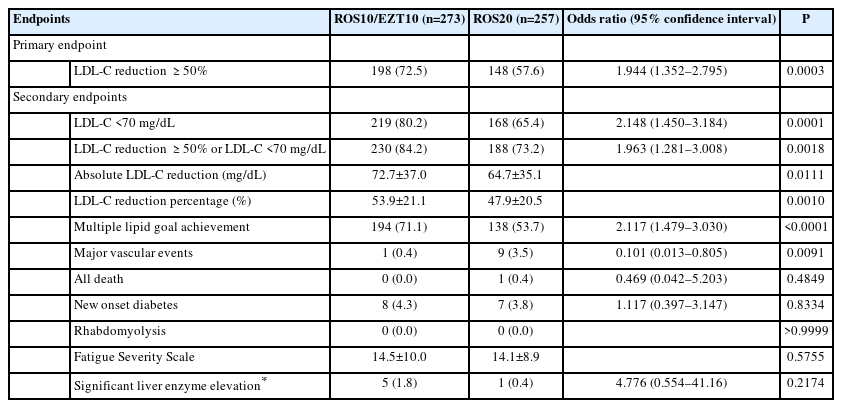
Of 584 randomized, 530 were included in the modified intention-to-treat analysis. The baseline LDL-C level was 130.2±34.7 mg/dL in the ROS10/EZT10 group and 131.0±33.9 mg/dL in the ROS20 group. The primary endpoint was achieved in 198 patients (72.5%) in the ROS10/EZT10 group and 148 (57.6%) in the ROS20 group (odds ratio [95% confidence interval], 1.944 [1.352–2.795]; P= 0.0003). LDL-C level <70 mg/dL was achieved in 80.2% and 65.4% in the ROS10/EZT10 and ROS20 groups (P=0.0001). Multiple lipid goal achievement rate was 71.1% and 53.7% in the ROS10/EZT10 and ROS20 groups (P<0.0001). Major vascular events occurred in 1 patient in the ROS10/EZT10 group and 9 in the ROS20 group (P=0.0091). The adverse event rates did not differ between the two groups.
Conclusion
Moderate-intensity rosuvastatin plus ezetimibe was superior to high-intensity rosuvastatin alone for intensive LDL-C reduction in patients with recent ischemic stroke. With the combination therapy, more than 70% of patients achieved LDL-C reduction ≥50% and 80% had an LDL-C <70 mg/dL at 90 days.
Introduction
In patients with ischemic stroke or transient ischemic attack due to atherosclerosis or having concomitant atherosclerotic diseases, intensive low-density lipoprotein cholesterol (LDL-C) lowering is recommended to reduce the risk of subsequent vascular events. In meta-analyses, the benefit of stroke prevention was greater as the magnitude of LDL-C reduction was larger and the achieved LDL-C level was lower [1,2]. For secondary stroke prevention, guidelines recommend LDL-C reduction of ≥50% from baseline or a target LDL-C goal of <70 mg/dL [3,4].
As a first-line strategy for intensive LDL-C lowering, high-intensity statins are generally recommended. However, high-intensity statins as compared to moderate- or low-intensity statins increase the risks of diabetes, hepatic dysfunction, and myopathy [5-7]. Furthermore, the additional reduction in LDL-C with increasing statin dose is limited, only 5% to 7% absolute reduction with doubling the dose of statins [8].
Non-statin lipid-lowering therapies added to statins were effective for preventing cardiovascular events, and the benefit was likely driven by further LDL-C lowering [9-12]. Ezetimibe inhibits intestinal cholesterol absorption by binding to the Niemann–Pick C1-like 1 (NPC1L1) protein [13]. When added to statins, ezetimibe resulted in a substantial LDL-C reduction, and the reduction appeared greater than that achieved with doubling statin dose [8,14,15]. For patients with recent ischemic stroke, there has been no randomized trial that compared moderate-intensity statin plus ezetimibe and high-intensity statin for LDL-C reduction.
We conducted the ROSuvastatin plus Ezetimibe Treatment for Target LDL-C goal Achievement in patients with recent ischemic Stroke (ROSETTA-Stroke) trial to evaluate moderate-intensity rosuvastatin plus ezetimibe, as compared with high-intensity rosuvastatin alone for target LDL-C goal achievement in patients with recent ischemic stroke of atherosclerotic origin or having concomitant atherosclerotic cardiovascular diseases.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Trial design
In this multicenter, randomized, open-label, parallel-group trial conducted at 13 sites in South Korea, we compared rosuvastatin 10 mg plus ezetimibe 10 mg once daily (ROS10/EZT10) and rosuvastatin 20 mg alone once daily (ROS20) for target LDL-C goal achievement in patients with recent ischemic stroke. In Korea, rosuvastatin 40 mg has not been approved, and accordingly we selected the dose of 20 mg as a high-intensity rosuvastatin and 10 mg as a moderate-intensity rosuvastatin [16].
This was an investigator-initiated trial and funded by Hanmi Pharm. Co., Ltd. The sponsor participated in the trial design and provided study medications, but it had no role in the conduct of the trial, data collection and analysis, and writing and submitting the manuscript for publication. All the data were collected by the site investigators, and were monitored and maintained by an independent research organization contracted by the principal investigator affiliated academic institution. All the authors vouch for the completeness and accuracy of the data and the fidelity of the trial to the protocol. The principal investigator and independent statisticians had full access to and vouch for the data and analysis. The trial was approved by local institutional review boards. Enrolled patients or their legally authorized representatives provided written informed consent. The trial is registered with ClinicalTrials.gov (NCT03993236).
Study population
Patients were eligible for enrollment if they were 19 years or older; they had recent ischemic stroke within 90 days confirmed by diffusion-weighted imaging; statin therapy was indicated according to the recommendations of the 2014 American Heart Association/American Stroke Association guidelines [17]; they had not taken statins within 4 weeks before the index stroke; and the baseline LDL-C level was measured after onset of the index stroke. Prior to randomization, administration of non-study statins immediately after acute stroke hospitalization according to each institution’s practice protocol was allowed. In this case, the baseline LDL-C level should be measured within 3 days of initiating non-study statins, and the randomization and initiation of study medications should be completed within 7 days of the baseline LDL-C level measurement. Detailed inclusion and exclusion criteria are provided in Supplementary Table 1.
Randomization, intervention, and follow-up
Eligible patients were randomly assigned, in a 1:1 ratio, to receive, once daily, either rosuvastatin 10 mg plus ezetimibe 10 mg (ROS10/EZT10 group) or rosuvastatin 20 mg alone (ROS20 group). Randomization was stratified by centers and baseline LDL-C levels (<100 mg/dL vs. ≥100 mg/dL), using an interactive web response system. All patients received the standard secondary stroke prevention therapy recommended by current practice guidelines. Concomitant use of additional lipid-lowering agents (non-study statins, fibrates, or omega-3 fatty acids) was not allowed during the trial.
Patients were scheduled for follow-up visits at 30 days and at 90 days after the enrollment. Unscheduled visits were made as needed. Major vascular events (including recurrent stroke, coronary event, or vascular death) and adverse events were collected during the follow-up. Lipid profiles and laboratory tests for monitoring adverse events were measured at the 90-day follow-up visit. For patients who withdrew during the trial, we recommended to measure lipid profiles at the time of withdrawal, which were used for endpoint analysis if available.
Endpoints
The primary endpoint was the proportion of patients who achieved a target LDL-C goal, defined as LDL-C reduction of ≥50% from baseline. We selected the LDL-C reduction of ≥50% as a target goal, which was widely recommended by the guidelines when we designed this trial [16].
Secondary endpoints included (1) proportion of patients achieving LDL-C level <70 mg/dL, (2) proportion of patients with LDL-C reduction ≥50% or achieved LDL-C level <70 mg/dL, (3) the absolute and percentage changes in LDL-C level, (4) proportion of patients achieving multiple lipid goals of total cholesterol <200 mg/dL, LDL-C <70 mg/dL, and triglyceride <150 mg/dL [18], (5) composite of major vascular events including stroke (ischemic or hemorrhagic), coronary event (myocardial infarction or coronary revascularization), or vascular death, (6) all cause of death, (7) new onset diabetes, (8) fatigue assessed by the Fatigue Severity Scale [19], (9) rhabdomyolysis, and (10) significant liver enzyme elevation (alanine aminotransferase or aspartate aminotransferase elevation >3 times from baseline).
Safety endpoints included any adverse event, treatment-emergent adverse event (TEAE), any serious adverse event, and serious TEAE. TEAEs were defined as adverse events for which a causal relationship with study medications could not be excluded. Serious events included those that (1) resulted in death or were life-threatening, (2) required or prolonged hospitalization, (3) resulted in persistent or significant disability/incapacity, (4) caused a congenital anomaly/birth defect, or (5) resulted in the development of drug dependency/abuse or significant medical events.
Sample size calculation and statistical analysis
This trial was designed to detect the superiority of rosuvastatin 10 mg plus ezetimibe 10 mg once daily over rosuvastatin 20 mg once daily for achieving LDL-C reduction ≥50% from baseline. Based on the prior study findings [20], the sample size was calculated by assuming that the primary endpoint rate would be 82% in the ROS10/EZT10 group and 72% in the ROS20 group. Under two-sided significance level of 5%, a total of 554 patients (277 patients per group) were needed to ensure 80% of power of detecting 10% of absolute difference in the primary endpoint rate using the chi-square test. Assuming a 5% dropout rate, a total of 584 patients (292 patients per group) was planned to enroll. Given that lipid-lowering with statins and ezetimibe are approved and widely used in daily clinical practice, neither an independent data safety monitoring board was organized, nor interim analysis was planned.
The primary and secondary endpoints were assessed in the modified intent-to-treat (ITT) population, which included patients who were randomized, received at least 1 dose of study medications, and completed follow-up LDL-C level measurement. For patients who withdrew during the trial after initiation of study medications, but their LDL-C levels were measured at the time of withdrawal, they were included in the modified ITT population. Per-protocol (PP) analysis was additionally conducted, which included patients who had no major protocol violation, took >80% of the assigned study medications, and did not take non-study lipid-lowering agents after enrollment among the modified ITT population. Adverse events were assessed in the safety population, which included all patients who were randomized and received at least 1 dose of study medications.
The primary endpoint was analyzed using the chi-square test. Additionally, an adjusted analysis was planned if there were significant differences in the baseline characteristics with biological plausibility, but no such variable was found. For the baseline LDL-C levels, we predetermined that they would not be adjusted even if there was a significant imbalance between the two groups because randomization was stratified by the baseline LDL-C levels (<100 mg/dL vs. ≥100 mg/dL). Secondary and safety endpoints were analyzed, using the chi-square test, Fisher’s exact test, Student’s t-test, or Mann-Whitney U-test as indicated in the statistical analysis plan (SAP). All statistical analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC, USA), and a two-sided P-value <0.05 was considered significant. All analyses were pre-planned in the SAP and performed by two independent statisticians who were blinded to the treatment allocation.
Results
Patients
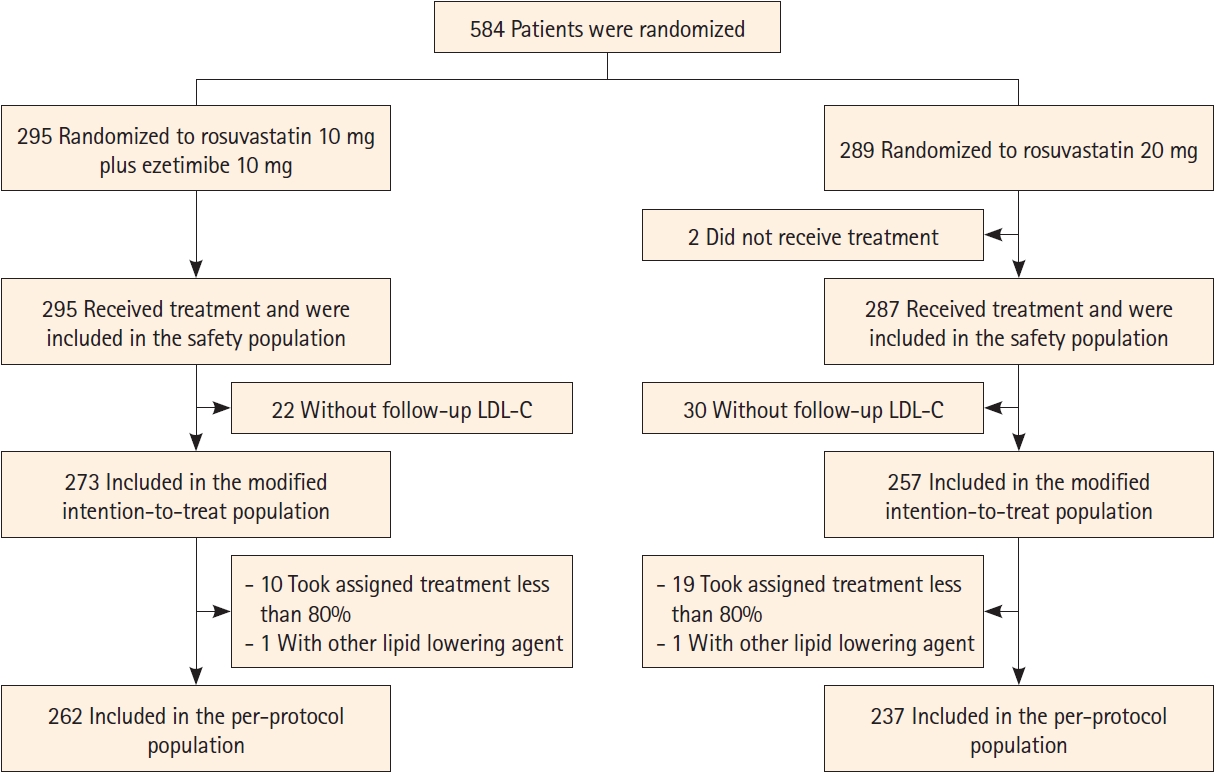
Between September 9, 2019, and September 27, 2021, 584 patients were randomized (295 to the ROS10/EZT10 group and 289 to the ROS20 group). After randomization, 2 patients in the ROS20 group did not take any study medication, and 52 (22 in the ROS10/EZT10 group and 30 in the ROS20 group) did not undergo follow-up LDL-C measurements. Therefore, 530 patients (273 in the ROS10/EZT10 group and 257 in the ROS20 group) were included in the modified ITT population. Of the modified ITT population, 31 patients took less than 80% of study medications or received other lipid-lowering agents, and 499 patients (262 in the ROS10/EZT10 group and 237 in the ROS20 group) were included in the PP population. The safety population included 582 patients (295 in the ROS10/EZT10 group and 287 in the ROS20 group) (Figure 1).
Baseline demographics and clinical characteristics were well balanced between the two groups (Table 1). The mean LDL-C level at baseline was 130.2 mg/dL in the ROS10/EZT10 group and 131.0 mg/dL in the ROS20 group. In each group, the median interval from the index ischemic stroke onset to randomization was 4 days, and the median National Institutes of Health Stroke Scale (NIHSS) score at randomization was 2. Before randomization, 193 patients (70.7%) in the ROS10/EZT10 group and 188 (73.2%) in the ROS20 group received non-study statins immediately after acute stroke hospitalization. The proportion of patients taking non-study statins before randomization and the average dose (converted to atorvastatin equivalent dose) did not differ between the two groups. The interval from initiating non-study statins to randomization was 3.0±1.7 days in the ROS10/EZT10 group and 3.1±1.6 days in the ROS20 group (P=0.8958) (Table 1 and Supplementary Table 2).
The follow-up LDL-C levels were obtained at 90 days in 516 patients (266 in the ROS10/EZT10 group and 250 in the ROS20 group). In 14 (7 in each group) patients, the follow-up LDL-C levels were measured at the time of withdrawal, and these values were used for the endpoint analysis.
Endpoints
The primary endpoint of LDL-C reduction ≥50% from baseline was achieved in 198 patients (72.5%) in the ROS10/EZT10 group and 148 (57.6%) in the ROS20 group of the modified ITT population, and the difference was significant (odds ratio [95% confidence interval], 1.944 [1.352–2.795]; P=0.0003) (Table 2). Of secondary endpoints regarding lipid profiles, the ROS10/EZT10 group compared to the ROS20 group had higher proportions of patients with the achieved LDL-C level <70 mg/dL (80.2% vs. 65.4%; OR [95% CI], 2.148 [1.450–3.184]; P=0.0001), LDL-C reduction ≥50% or the achieved LDL-C <70 mg/dL (84.2% vs. 73.2%; OR [95% CI], 1.963 [1.281–3.008]; P=0.0018), and multiple lipid goal achievement (71.1% vs. 53.7%; OR [95% CI], 2.117 [1.479– 3.030]; P<0.0001). The absolute LDL-C reduction was greater in the ROS10/EZT10 group than in the ROS20 group (72.7 mg/dL vs. 64.7 mg/dL; P=0.0111), and accordingly the achieved LDL-C level was lower in the ROS10/EZT10 group than in the ROS20 group (57.4±24.6 mg/dL vs. 66.3±26.2 mg/dL; P<0.0001) (Supplementary Table 3).
Major vascular events occurred in 1 patient (0.4%) in the ROS10/EZT10 group and 9 (3.5%) in the ROS20 group (OR [95% CI], 0.101 [0.013–0.805]; P=0.0091). Of the 10 major vascular events, 7 were recurrent ischemic strokes and 3 were coronary events. Death occurred in 1 patient (sudden cardiac death) in the ROS20 group. There was no rhabdomyolysis during the trial. The two groups did not differ in the incidences of new onset diabetes and significant liver enzyme elevation, and the Fatigue Severity Scale. The results of the PP population analyses were consistent with those of the modified ITT population analyses (Supplementary Table 4).
Total cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were comparable between the two groups at baseline (Table 1). At follow-up, the total cholesterol level was lower in the ROS10/EZT10 group than in the ROS20 group. However, the 90-day high-density lipoprotein cholesterol and triglyceride levels did not differ between the two groups (Supplementary Table 3).
Of the modified ITT population, 14 patients (4 in the ROS10/EZT10 group and 10 in the ROS20 group) discontinued study medications transiently or permanently between the 1st followup visit (at day 30) and the final visit (at day 90). Analysis excluding these patients showed consistent results regarding LDL-C related endpoints (Supplementary Table 5). Additional analysis excluding 22 patients (8 in the ROS10/EZT10 group and 14 in the ROS20 group) who transiently or permanently discontinued study medications at any point during the trial also showed similar results (Supplementary Table 6).
Subgroup analyses were not prespecified, but were undertaken in a post-hoc manner. For the primary endpoint, treatment effects were consistent across major subgroups of age (<64 vs. ≥64), sex, baseline LDL-C level (<100 mg/dL vs. ≥100 mg/dL), hypertension, diabetes, and smoking (Figure 2).
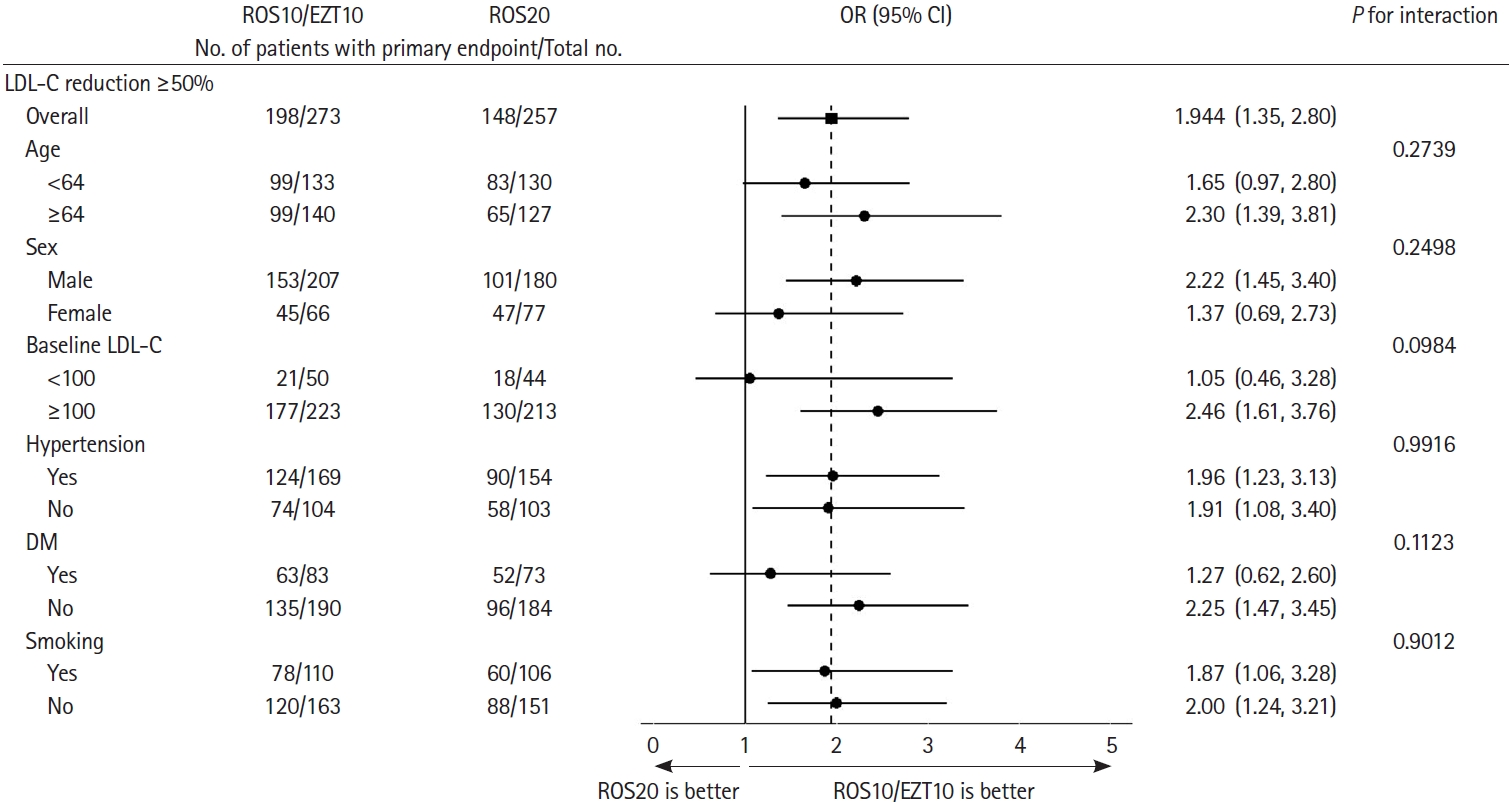
Subgroup analyses of primary endpoint, according to post hoc subgroups. LDL-C reduction ≥50% (the primary end point) at 90 days among patients in the ROS10/EZT10 group and in the ROS20 group, according to post hoc subgroups. LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus.
Adverse events
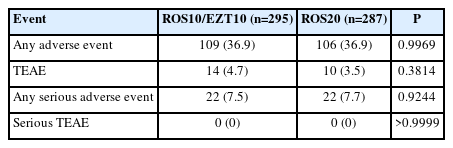
In the safety population, there were no significant differences between the ROS10/EZT10 and ROS20 groups in the rates of any adverse event (36.9% vs. 36.9%; P=0.9969), any TEAE (4.7% vs. 3.5%; P=0.3814), any serious adverse event (7.5% vs. 7.7%; P=0.9244), and serious TEAE (0% vs. 0%; P>0.9999) (Table 3). Details of any serious adverse events and TEAE according to individual organs are described in Supplementary Table 7 and 8. For muscle-related adverse events, there was no clinically significant myopathy. Myalgia occurred in 5 patients (2.3%) in the ROS10/EZT10 group and 3 (1.4%) in the ROS20 group (P=0.5000). Of these, myalgia in 6 patients (3 in each group) was considered as TEAEs.
Discussion
In this randomized trial, we found that the combination of moderate-intensity rosuvastatin 10 mg plus ezetimibe 10 mg was superior to high-intensity rosuvastatin 20 mg alone for LDL-C reduction in patients with recent ischemic stroke who initiated lipidlowering therapy. With the combination therapy, more than 70% of patients achieved an LDL-C reduction ≥50% from baseline, and more than 80% had their LDL-C levels <70 mg/dL at 90 days. For every 100 patients indicated for LDL-C lowering, approximately additional 15 patients would achieve these target LDL-C goals with the combination therapy as compared to rosuvastatin 20 mg alone. In addition, 71% of patients with the combination therapy achieved the multiple lipid goals, an absolute increase of about 17% as compared to that with rosuvastatin 20 mg alone.
Our findings are in accord with earlier studies. A prior meta-analysis showed that the LDL-C reduction was greater with adding ezetimibe to ongoing statins (26.0% reduction from baseline) than with doubling the dose of ongoing statins (9.7% reduction) [15]. In the randomized comparison of efficacy and safety of lipid-lowering with statin monotherapy versus statin ezetimibe combination for high-risk cardiovascular disease (RACING) conducted in South Korea, 73% of patients receiving rosuvastatin 10 mg plus ezetimibe 10 mg and 55% of those receiving rosuvastatin 20 mg alone achieved an LDL-C level of <70 mg/dL at 1 year [21]. The difference in the proportion (about 18%) between the 2 arms in RACING was quite comparable to that observed in our trial (about 15%). Both RACING and our trial would indicate that 7–8 out of 10 Korean patients with atherosclerotic diseases could achieve an LDL-C level of <70 mg/dL with rosuvastatin 10 mg plus ezetimibe 10 mg once daily.
In this trial, the clinical endpoints of major vascular events occurred significantly less in the ROS10/EZT10 group than in the ROS20 group. However, this trial was neither designed nor powered to detect the difference in the clinical endpoint. Given the short trial period and the magnitude of the achieved LDL-C level difference between the two groups, the clinical benefit observed in this study is highly likely due to by chance. Meta-analyses and a clinical trial indicated the greater benefit for secondary stroke prevention with greater LDL-C reduction or lower LDL-C levels achieved [1,2,22], and accordingly moderate-intensity rosuvastatin plus ezetimibe could be superior to high-intensity rosuvastatin to prevent vascular events. The RACING trial demonstrated that rosuvastatin 10 mg plus ezetimibe 10 mg was non-inferior to rosuvastatin 20 mg alone for the 3-year clinical composite outcome of major vascular events. The rate was slightly lower with the combination therapy (9.1%) than with rosuvastatin alone (9.9%) [21]. Therefore, both RACING and our trial suggest that rosuvastatin 10 mg plus ezetimibe 10 mg compared to rosuvastatin 20 mg alone was, at least, non-inferior for preventing vascular events, and was better for intensive LDL-C reduction. Clinical efficacy of statin plus ezetimibe combination therapy was further supported by a post hoc analysis of the Treat Stroke to Target (TST) trial [23]. In the lower LDL-C target group (LDL-C <70 mg/dL), the proportion of patients on combination therapy increased from <5% at baseline to >20% at 6 months and >50% at 3 years. The risk reduction of primary endpoint (major cardiovascular event) in the lower target group as compared with the higher target group (LDL-C 100±10 mg/dL) was significant during combination therapy, but not during statin monotherapy.
We observed a numerically higher incidence of alanine aminotransferase or aspartate aminotransferase elevation >3 times from baseline in the ROS10/EZT10 group than in the ROS20 group. However, the incidence was low and did not significantly differ between the two groups. There was no case of clinically significant hepatic dysfunction. The low TEAE incidences and no case of serious TEAE in the two groups support the safety of the two treatments.
In this trial, we selected rosuvastatin 20 mg as a high-intensity statin strategy because rosuvastatin 40 mg has not been approved for clinical use in Korea. The 2013 American College of Cardiology/American Heart Association guidelines categorized rosuvastatin 20 mg as high-intensity statin [16]. Studies have shown an increased risk of intracerebral hemorrhage with high-intensity statins, and the risk could be greater during the acute stage of ischemic stroke. However, in a randomized Korean trial involving 316 patients with acute ischemic stroke within 48 hours, the incidence of any intracranial hemorrhage detected on follow-up gradient-recalled echo magnetic resonance imaging at 14 days was not higher, but rather lower, in the rosuvastatin 20 mg group than in the placebo group [24]. In our study, patients were enrolled, on average, 4–5 days after acute ischemic stroke, and there was no hemorrhagic stroke during the follow-up. Therefore, both the combination therapy and high-intensity rosuvastatin appear safe in patients with acute ischemic stroke with regard to intracerebral hemorrhage.
Our study has several limitations. This was an open-label study, which was at risk of a reporting bias for clinical events. However, key endpoints were objective laboratory results, and independent statisticians blinded to the treatment allocation analyzed the data. We exclusively enrolled Korean patients. The response to lipid-lowering therapy could differ across ethnicities [25], and thereby the generalizability of our findings might be limited. In addition, most of the included patients had a mild stroke, which could limit the applicability of our findings to patients with more severe strokes. Whether the greater LDL-C reduction with the combination therapy versus high-intensity rosuvastatin alone could be translated into better secondary stroke prevention remains unclear, which should be formally tested by a well-designed large trial.
Conclusions
The ROSETTA-Stroke trial showed that moderate-intensity rosuvastatin plus ezetimibe was superior to high-intensity rosuvastatin alone for intensive LDL-C lowering in patients with recent ischemic stroke who initiated lipid-lowering therapy. For every 100 patients treated with rosuvastatin 10 mg plus ezetimibe 10 mg once daily, almost 73 achieved LDL-C reduction ≥50%, and about 80 had an LDL-C level <70 mg/dL at 90 days.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.02957.
Inclusion and exclusion criteria
Non-study statin use before randomization
ipid profiles at follow-up in the modified intention- to-treat population
End points in the per-protocol population
LDL-C related endpoints after excluding patients who discontinued study medications transiently or permanently between the 1st follow-up visit (at day 30) and the final visit (at day 90)
LDL-C related endpoints after excluding patients who trainsiently or permanently discontinued study medications during the trial
Any serious adverse events by MedDRA body system in the safety population
TEAEs by MedDRA body system in the safety population
Notes
Funding statement
This study was funded by Hanmi Pharm. Co., Ltd.
Conflicts of interest
K.- S. Hong, O. Y. Bang, J.-H. Park, J.-M. Jung, S.-H. Lee, T.-J. Song, H. S. Nam, H.-K. Park, K.-H. Jung, S. H. Heo, J. Koo, K.-H. Yu, K.-Y. Park, C. K. Kim, H.-K Park, J. Lee, W.-K. Seo received research grants from Hanmi Pharm. Co., Ltd.
Author contribution
Conceptualization: KSH, OYB, HKP, WKS. Study design: KSH, OYB, HKP, WKS, JL (Juneyoung Lee). Methodology: KSH, OYB, HKP, WKS, JL (Juneyoung Lee). Data collection: all authors. Investigation: all authors. Statistical analysis: KSH, OYB. JL (Jiyoon Lee), JL (Juneyoung Lee). Writing—original draft: KSH, JL (Juneyoung Lee). Writing—review & editing: all authors. Funding acquisition: KSH, OYB. Approval of final manuscript: all authors.