 |
 |
- Search
| J Stroke > Volume 25(1); 2023 > Article |
|
Abstract
Background and Purpose
Nelonemdaz (Neu2000) has both selective antagonism against 2B subunit of N-methyl-D-aspartate receptor and antioxidant activity. This drug provides sufficient evidence of neuroprotection in acute cerebral ischemia/reperfusion models. This phase III trial aims to determine this effect in patients.
Design
The Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz is a multicenter, double-blinded clinical trial. A total of 496 patients will be randomly assigned into the nelonemdaz (a total of 5,250 mg divided by 10 times for 5 days) and placebo groups. Patients will be included if they have an acute ischemic stroke (National Institutes of Health Stroke Scale score ≥8) caused by intracranial large vessel occlusion in the anterior circulation (Alberta Stroke Program Early CT Score ≥4), and if they are expected to undergo endovascular thrombectomy within 12 hours after stroke onset.
Endpoints
The primary endpoint is a favorable shift in the modified Rankin Scale (mRS) score at 90 days after the first dose of drug. The data will be analyzed by the Cochran-Mantel-Haenszel shift test. The secondary endpoints include functional independence (mRS 0-2) at 35 and 90 days, the favorable shift of mRS at 35 days, the proportion of mRS 0 at 35 and 90 days, and the occurrence rates of symptomatic intracranial hemorrhage within 7 days.
Endovascular thrombectomy (EVT) for acute ischemic stroke is beneficial in patients with acute ischemic stroke caused by large vessel occlusion. Five landmark clinical trials showed that EVT increases the substantial reperfusion rate by approximately 70%-80% and significantly decreased the risk of disability or death at 90 days compared to the control group [1-5]. However, EVT cannot prevent impairments in the ability to live independently in approximately half of patients with acute ischemic stroke [6]. Irreversible ischemic brain injury that has already occurred prior to reperfusion therapy plays a major role in contributing to disability in such cases. Moreover, the ischemic injury may continue even after reperfusion therapy because of incomplete reperfusion or reperfusion injury [7-9].
Nelonemdaz (Neu2000), a novel synthetic derivative of aspirin and sulfasalazine, selectively inhibits the 2B subunit of the glutamate N-methyl-D-aspartate (NMDA) receptor (NR2B) and is a potent scavenger of reactive oxygen species that plays a major role in reperfusion injury after thrombectomy [10,11]. Therefore, nelonemdaz may prevent neuronal injury after EVT by blocking NMDA-receptor-mediated neurotoxicity and free radical injury [12-14].
A previous phase II clinical trial with nelonemdaz revealed its favorable potential to improve clinical outcomes after EVT for acute ischemic stroke with large vessel occlusion without causing serious side effects [15]. The Rescue On reperfusion Damage in cerebral Infarction by Nelonemdaz (RODIN) trial aims to prove the efficacy and safety of nelonemdaz after EVT in acute ischemic stroke with intracranial large vessel occlusion by preventing ischemic and reperfusion injury.
We hypothesize that nelonemdaz treatment significantly improves the functional outcomes of the patients who have undergone EVT for acute ischemic stroke with large vessel occlusion, as measured by the 90-day modified Rankin Scale (mRS) score, in comparison with placebo treatment.
This is a phase III, multicenter, randomized, double-blind, placebo-controlled trial, which will be performed at over 20 stroke centers in South Korea. The study protocol has been approved by the South Korea Ministry of Food and Drug Safety and subsequently approved by the Institutional Review Board of each participating center. Written informed consent will be obtained from the enrolled patients or their legal guardians. The study flow is outlined in Figure 1. This study has been registered at ClinicalTrials.gov (NCT05041010).
The inclusion and exclusion criteria are listed in Table 1. Adult patients (≥19 years old) who have acute ischemic stroke caused by intracranial large vessel occlusion (internal carotid artery, or M1 or M2 segments of the middle cerebral artery by baseline angiography) and for whom the beginning of EVT is expected to begin within 12 hours after the last normal time are candidates for the current trial. Inclusion criteria include baseline National Institutes of Health Stroke Scale (NIHSS) score ≥8; pre-stroke mRS 0 or 1; and baseline Alberta Stroke Program Early CT Scores (ASPECTS) ≥4 on axial brain computed tomography (CT) or diffusion-weighted magnetic resonance imaging (MRI). Patients will be excluded if the intracranial vessel occlusions that cause acute ischemic stroke occur bilaterally. Moreover, patients would be excluded if they possess occlusions in the posterior circulation that causes acute ischemic stroke. Additionally, patients with serious medical conditions will be excluded.
Participants will be randomly assigned in a 1:1 ratio to one of two arms. After the stratification of the participating centers, centralized stratified block randomization will be used to randomly assign the participants into the nelonemdaz and placebo groups.
An interactive web-response system will be used for randomization. The participants will be assigned to each treatment group according to the allocation codes provided by the random assignment program, and the seeds will be assigned to the random assignment codes to enable reproducibility.
The participants, investigators, and sponsors are blinded to the treatments. In the context of drug administration, the site investigators will check the assigned randomized drug number in the order of the serial number, prepare the drug, and administer it to the participants. All data collected from each participant and the individual blinding codes will not be broken for pre-planned data analysis until the database has been locked.
An investigator will initiate the first infusion of the assigned dose in a double-blind manner. To optimize the results, the first drug should be infused as soon as possible after obtaining informed consent. Although we intend to administer the first infusion prior to reperfusion, infusion of the first dose will be considered up to 60 minutes after the groin puncture for the endovascular reperfusion treatment. After the infusion of the first dose of the study medication, nine more doses will be given every 12 hours. In the treatment arm, 750 mg of nelonemdaz will be infused as the first dose, followed by successive doses of 500 mg (Figure 1).
The primary endpoint is a favorable shift in the mRS score at 90 days after the first dose of the clinical trial drug. The secondary endpoints include the proportion of mRS 0-2 and mRS 0, respectively, at 35 and 90 days after the first dose of the clinical trial drug, the distribution of mRS at 35 days, NIHSS score 0-4 within 24 hours at 35 and 90 days, and Barthel Index ≥95 at 35 and 90 days. Infarct volume will be measured on brain MRI (alternatively, brain CT) at 7 and 90 days. Symptomatic intracranial hemorrhage within 7 days, which is defined as brain image-confirmed intracranial hemorrhage combined with neurological deterioration (NIHSS score increase ≥4), will also be evaluated.
Safety endpoints will include adverse and serious adverse events, all mortality up to 90 days, abnormal vital signs and physical examination signs, abnormal laboratory results including blood tests, urinalysis, electrocardiography and chest radiography, and any major generalized hemorrhage based on the definition of International Society of Thrombosis and Hemostasis (fatal hemorrhage; symptomatic hemorrhage in dangerous areas or organs, such as intraspinal, intraocular, peritoneal, intraarticular, pericardial, or intramuscular hemorrhage accompanied by compartment syndrome; or a reduction of ≥2 g/dL hemoglobin concentration or hemorrhage that necessitates transfusion of the packed red blood cells or whole blood ≥2 units) [16]. All serious adverse events will be reviewed by an independent case adjudication committee.
Clinical evaluations will include the measurement of the mRS score [17], Barthel Index [18], and NIHSS score [19]. These assessments will be conducted by blinded personnel in person at the outpatient clinic. However, the functional status can be assessed by telephone interviews with patients or their caregivers according to a standardized protocol if the patient cannot visit due to severe disability or death. Study evaluations are summarized in Table 2.
The core imaging lab will conduct blinded independent assessments of CT and MRI findings. The CT core lab will assess the baseline ASPECTS and the occlusion location in the cervicocephalic vessels. The MRI core lab will assess the infarct volume on axial brain MRI at 7 days and 90 days (the last axial brain CT to be performed at this point if MRI is not performed). The angiography core lab will assess the final reperfusion status after EVT. Expanded treatment in cerebral ischemia (eTICI) grade will be used to evaluate the reperfusion status, and eTICI grade ≥2b50 will be regarded as successful reperfusion.
For each of the subgroups below, multivariable analysis will be performed when the number of study participants is reliably obtained by subgroups. However, the multivariable analysis results for each subgroup are expected to have low statistical power due to the limitation of the analysis target, so the interpretation should be limited in descriptive aspects. Subgroup analyses will include the following: age (higher/less than 70 years); sex; NIHSS score (higher/less than the median); diabetes mellitus; ASPECTS score (higher/less than 7); MRI-based versus CT-based randomization; intravenous recombinant tissue plasminogen activator (rt-PA) infusion; eTICI grade (0-2a/2b/2c-3); and Trial of ORG 10172 in Acute Stroke Treatment (TOAST; cardioembolic/non-cardioembolic).
This study was designed to determine the superiority of nelonemdaz over placebo for the reduction of stroke-related disability in patients with acute ischemic stroke who have undergone EVT. The primary endpoint is a favorable shift in the mRS score at 90 days. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated using an ordinal logistic regression model, assuming a common OR (cOR) across all mRS cutoff points, and are provided only as an estimate of the treatment effect. As a result of the previous phase II trial, the odds for improvement integrated across all cutoff-points of the scale were increased by nelonemdaz, as compared with placebo (cOR=1.71; 95% CI, 0.90-3.25). Thus, we assume a cOR of 1.65 for nelonemdaz versus placebo. The sample size was calculated based on a two-sided superiority test with 80% power and a significance level of 0.05. We intend to enroll 496 patients, assuming a 20% dropout rate.
The primary efficacy will be assessed using a full analysis set (FAS), which includes all randomly assigned patients who have received at least one dose of the study drug and have undergone measurement of the primary endpoint. Per-protocol analysis will be additionally performed in patients with no major protocol violation for the inclusion and exclusion criteria, and a compliance of 80% or more. For the conclusion of the trial, we will use the FAS analysis.
The primary endpoint analysis will be performed using the Cochran-Mantel-Haenszel (CMH) shift test and an ordinal logistic regression model. The secondary endpoint analysis will be performed using the chi-square test, Fisher’s exact test, CMH shift test, Student’s t-test, and Wilcoxon rank sum test according to the type of variables.
The steering committee, which comprises several principal investigators from individual centers who are experts in stroke, cerebrovascular diseases, neurointervention, and biostatistics, will oversee all clinical trial conduct, and protocol development and amendment. GNT Pharma is the trial sponsor and funding source. The South Korea Ministry of Food and Drug Safety has approved the protocol as a phase III clinical trial for a new investigational drug. A contract research organization (CRO) will assess whether all trial procedures are being appropriately conducted, check whether the participants are included per protocol, and ensure that patient data have not been lost and have been collected well. For data and safety monitoring, the principal investigator (or a delegated investigator)—as a representative of the data and safety group—together with members of the CRO will review and manage the collected research data once a month. This would ensure continuous monitoring and data integrity as well as safety of the research participants. If an unexpected significant adverse event occurs, the drug administration can be discontinued at any time at the investigators’ discretion, and this will be reported to the sponsor and the Institutional Review Board.
Early clinical trials for neuroprotective molecules targeting either NMDA receptor-mediated excitotoxicity or oxidative stress have failed to identify beneficial effects in patients with acute ischemic stroke despite promising results in animal experiments [20]. Delayed delivery of neuroprotective drugs in clinical trials in comparison with preclinical studies might have caused these failures. Extensive evidence implies that excess activation of NMDA receptors underlies rapidly evolving fulminant neuronal cell necrosis over hours after an ischemic attack, which is accompanied by delayed neuronal and glial cell death occurring over hours and days through excess oxidative stress and apoptosis after reperfusion. The latter is unveiled by the administration of NMDA receptor antagonists, suggesting that blocking a single pathogenic pathway may be insufficient to achieve clinical benefit in ischemic-reperfusion injury. Since 2015, EVT has been approved for patients with acute ischemic stroke and large vessel occlusion, and a large portion of patients with the large vessel occlusion are reportedly reperfused by the treatment [6,21]. Patients who have similar conditions as the ischemia/reperfusion model can be enrolled, and neuroprotection treatment should be revisited [22]. It is conceivable to reason that a multi-target neuroprotection strategy appeals for a maximal prevention of ischemic-reperfusion injury. In this context, nelonemdaz was designed as a dual neuroprotectant that targets both the NMDA receptor and free radicals, the two major drivers of post-stroke neuronal death (Figure 2A) [7-9].
Previously tested NMDA antagonists have been nonselective and potentially too potent, leading to problematic side effects (e.g., psychosis) in humans, possibly accompanied by enhancement in delayed neuronal apoptosis [23,24]. Early clinical trials using NMDA receptor blockers reported higher mortality rates in the treatment group than in the placebo group [25,26], and reported psychological adverse effects among the treated patients [25]. Glutamate receptors purportedly have been proposed to have both pro-survival and pro-death signaling [27]. More specifically, the 2A subunit of the NMDA receptor (NR2A) may have both signaling pathways whereas NR2B may favor a pro-death signaling pathway [8]. Thus, a selective blocking NR2B would be theoretically more effective and safer for the treatment of acute cerebral infarction. The glutamate NR2B-postsynaptic density-95-neuronal nitric oxide synthase (GluN2B-PSD-95-nNOS) complex is one of the downstream death signaling pathways from NR2B, and nerinetide (NA1, Tat-NR2B9c) interferes with the PSD-95 so that the interaction between PSD-95 and NR2B is perturbed. In such a scenario, the glutamate toxicity can be ameliorated [8]. However, unfortunately, the phase III clinical trial, Safety and Efficacy of Nerinetide (NA-1) in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE-NA1), has failed to show the efficacy of the drug. Again, a new clinical trial, Efficacy and Safety of Nerinetide in Participants With Acute Ischemic Stroke Undergoing Endovascular Thrombectomy Excluding Thrombolysis (ESCAPE-NEXT), is currently ongoing with a revised protocol, considering a drug interaction with alteplase [28].
Nelonemdaz attenuates NMDA-induced currents as a reversible, noncompetitive, and activity-dependent NMDA receptorgating modifier that was revealed to be more specific to NR2B but not to NR2A [11]. The unblocking rate of nelonemdaz from the nelonemdaz-NMDA receptor complex is approximately eight times faster than that of memantine, a moderate NMDA receptor antagonist approved for the treatment of Alzheimer’s disease. These characteristics indicate its additional safety in comparison to high affinity NMDA receptor antagonists that produce neurotoxicity and psychotic symptoms. Neurotoxicity produced by dizocilpine (MK-801), a nonselective NMDA receptor antagonist, is not observed in rodents exposed to supratherapeutic doses of nelonemdaz [10]. In two phase I clinical trials encompassing a total of 165 healthy subjects conducted in the USA and China, administration of nelonemdaz up to 6,000 mg was well tolerated. It was also devoid of any psychological symptoms associated with previous NMDA receptor antagonist drug candidates [12]. A recently published phase II clinical trial for nelonemdaz, Safety and Optimal Neuroprotection of neu2000 in Ischemic Stroke With Endovascular reCanalizion (SONIC), also revealed no serious adverse effects including psychological symptoms [15].
After attenuating glutamate toxicity, nelonemdaz scavenges free radicals in both downstream of NMDA receptor-related excitotoxicity and reperfusion injury (Figure 2B). This is the reason for administering a high dose of nelonemdaz in the beginning, followed by nine successive moderate doses of the drug for both targets. Nelonemdaz effectively controlled free radical production and neuronal death than therapeutic and investigational antioxidants including edaravone, NXY-059, and Trolox in cultured cortical cells exposed to prooxidants such as Fe2+ and buthionine sulfoximine, a glutathione-depleting agent [10]. Nelonemdaz directly and potently removes free radicals as a spin-trapping molecule and displays scavenging activity against hydroxyl radicals, superoxide, nitric oxide, and peroxynitrite [12,13]. The administration of nelonemdaz prevents the production of slowly evolving free radicals in brain regions that undergo neurodegeneration following transient focal cerebral and forebrain ischemia [14,29].
The study design of the RODIN trial, which was based on the results of the phase II SONIC trial, is similar to but slightly different from the ESCAPE-NA1 and ESCAPE-NEXT trials of nerinetide (Table 3) [28,30]. The participant characteristics were almost identical, since both trials included patients with acute ischemic stroke caused by intracranial large vessel occlusion and underwent EVT. The inclusion of neurological deficits and ASPECTS volume may indicate slightly greater disease severity in the RODIN trial than in the ESCAPE-NEXT trial. Moreover, while the ESCAPE-NEXT will involve the administration of a single intravenous infusion of nerinetide, nelonemdaz will be infused intravenously 10 times over 5 days (5,250 mg total) to prevent delayed reperfusion injury in the RODIN trial.
In summary, nelonemdaz targets (1) selective NR2B, the activation of which causes Na+ and Ca++ influx into neuronal cells and then induces downstream death-signaling pathways, and (2) free radicals, which are released from the downstream pathways of glutamate receptors and subsequent reperfusion injury. In the RODIN phase III trial, the first high dose will be infused for the former target, and then the second to tenth moderate doses will be infused for the latter target. Therefore, the results of this study will show that nelonemdaz treatment improves the functional outcome of acute ischemic stroke patients undergoing reperfusion therapy by the preventing neuronal injury during acute ischemic conditions and prolonged free radical mediated neuronal injury after reperfusion.
Notes
Disclosure
Jin Soo Lee and Dennis W. Choi serve as advisory board members of GNT Pharma, and Ji Sung Lee is a statistical consultant to GNT Pharma. Byoung Joo Gwag is the Chief Executive Officer and Chun San An is the Clinical Development Officer of GNT Pharma. All remaining authors have declared no conflicts of interest.
Figure 1.
Trial flowsheet. NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; M1, M1 segment of middle cerebral artery; M2, M2 segment of middle cerebral artery; ASPECTS, Alberta Stroke Program Early CT Score; MRI, magnetic resonance imaging.

Figure 2.
Pathomechanism of acute cerebral infarction and pharmacological actions of nelonemdaz. (A) Excessive glutamates in the synaptic cleft activate N-methyl-D-aspartate (NMDA) receptor, and excitotoxicity subsequently occurs. Oxidative stress, also causing neuronal damage, is sustained especially by reperfusion injury. (B) Nelonemdaz noncompetitively blocks the 2B subunit of NMDA receptor, the activation of which causes fast excitotoxicity. This also results in scavenging free radicals, which are involved from slow oxidative stress. The first high dose of nelonemdaz targets the fast excitotoxicity and the successive moderate doses aids in decreasing oxidative stress during the Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz trial.
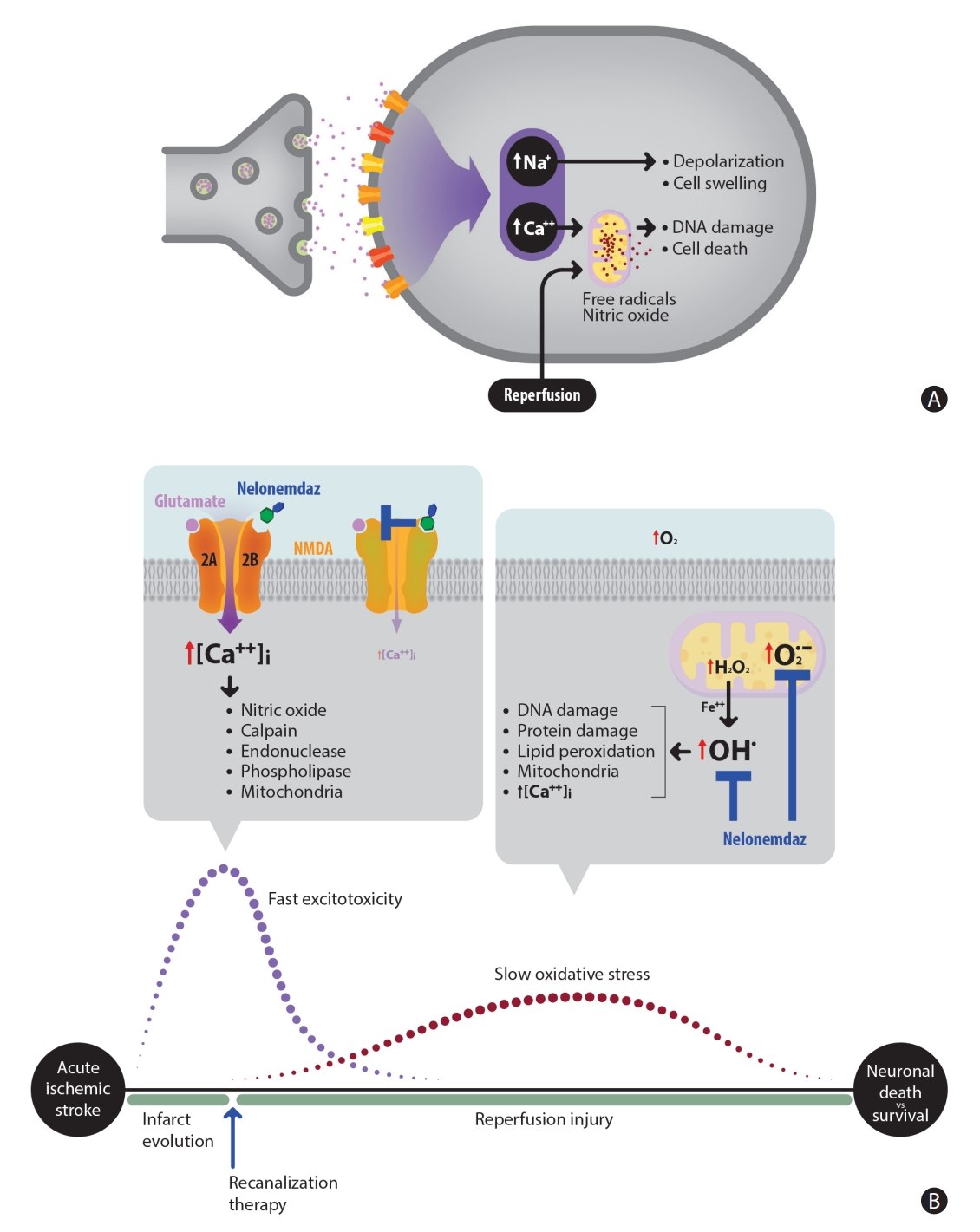
Table 1.
Inclusion and exclusion criteria of RODIN study
RODIN, Rescue On reperfusion Damage in cerebral Infarction by Nelonemdaz; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; CTA, computed tomography angiography; MRA, magnetic resonance angiography; ASPECTS, Alberta Stroke Program Early CT Score; CT, computed tomography; MRI, magnetic resonance imaging.
Table 2.
Visits and evaluations for the RODIN trial
Table 3.
Protocol comparison among neuroprotective trials
| ESCAPE-NA1 [28] | ESCAPE-NEXT [30] | SONIC [15] | RODIN | ||
|---|---|---|---|---|---|
| Outcomes | |||||
| Primary endpoint | mRS 0-2 | mRS 0-2 | mRS 0-2 | Favorable shift of mRS | |
| Inclusion criteria | |||||
| Pre-stroke | BI ≥95 | BI ≥95 | BI >90 | mRS 0-1 | |
| Occlusion site | Intracranial ICA, MCA M1 (M2) | Intracranial ICA, MCA M1 (M2) | Intracranial ICA, MCA M1 | Intracranial ICA, MCA M1, M2 | |
| Onset to EVT | ≤12 h | ≤12 h | ≤8 h | ≤12 h | |
| NIHSS | ≥6 | ≥6 | ≥8 | ≥8 | |
| ASPECTS | >4 | >4 | >5 | >3 | |
| Exclusion criteria | |||||
| IV tPA | Not considered | Exclusive | Not considered | Not considered | |
| Collateral | If absent | If absent | If poor | Not considered | |
| Complete reperfusion | Not considered | Exclusive | Not considered | Not considered | |
| Enrollment | |||||
| Subjects | 556+549=1,105 | 510+510=1,020 (on recruiting) | 71+71+67=209 | 298+298=496 (on recruiting) | |
ESCAPE-NA1, Safety and Efficacy of Nerinetide (NA-1) in Subjects Undergoing Endovascular Thrombectomy for Stroke; ESCAPE-NEXT, Efficacy and Safety of Nerinetide in Participants With Acute Ischemic Stroke Undergoing Endovascular Thrombectomy Excluding Thrombolysis; SONIC, Safety and Optimal Neuroprotection of neu2000 in Ischemic Stroke With Endovascular reCanalizion; RODIN, Rescue On reperfusion Damage in cerebral Infarction by Nelonemdaz; mRS, modified Rankin Scale; BI, Barthel Index; ICA, internal carotid artery; MCA, middle cerebral artery; EVT, endovascular thrombectomy; NIHSS, National Institute of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; IV, intravenous; tPA, tissue plasminogen activator.
References
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20.

2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-1030.


3. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-2295.


4. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-2306.


5. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-1018.


6. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-1731.


7. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999;22:391-397.


8. Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain 2018;11:15.



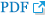
9. Choi DW. Excitotoxicity: still hammering the ischemic brain in 2020. Front Neurosci 2020;14:579953.



10. Gwag BJ, Lee YA, Ko SY, Lee MJ, Im DS, Yun BS, et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J Cereb Blood Flow Metab 2007;27:1142-1151.


11. Noh J, Lee ES, Chung JM. The novel NMDA receptor antagonist, 2-hydroxy-5-(2,3,5,6-tetrafluoro-4-trifluoromethyl-benzylamino)-benzoic acid, is a gating modifier in cultured mouse cortical neurons. J Neurochem 2009;109:1261-1271.


12. Cho SI, Park UJ, Chung JM, Gwag BJ. Neu2000, an NR2B-selective, moderate NMDA receptor antagonist and potent spin trapping molecule for stroke. Drug News Perspect 2010;23:549-556.


13. Visavadiya NP, McEwen ML, Pandya JD, Sullivan PG, Gwag BJ, Springer JE. Antioxidant properties of Neu2000 on mitochondrial free radicals and oxidative damage. Toxicol In Vitro 2013;27:788-797.


14. Im DS, Jeon JW, Lee JS, Won SJ, Cho SI, Lee YB, et al. Role of the NMDA receptor and iron on free radical production and brain damage following transient middle cerebral artery occlusion. Brain Res 2012;1455:114-123.


15. Hong JM, Lee JS, Lee YB, Shin DH, Shin DI, Hwang YH, et al. Nelonemdaz for patients with acute ischemic stroke undergoing endovascular reperfusion therapy: a randomized phase II trial. Stroke 2022;53:3250-3259.



16. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-694.


17. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091-1096.


18. Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke 2011;42:1146-1151.


19. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-870.


20. Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke 2012;7:407-418.


21. Lee JS, Lee SJ, Hong JM, Choi JW, Hong JH, Chang HW, et al. Temporal changes in care processes and outcomes for endovascular treatment of acute ischemic stroke: retrospective registry data from three Korean centers. Neurointervention 2018;13:2-12.



22. Lee JS, Hwang YH, Sohn SI. Factors contributing to an efficacious endovascular treatment for acute ischemic stroke in Asian population. Neurointervention 2021;16:91-110.



23. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995;52:998-1007.


24. Gwag BJ, Lobner D, Koh JY, Wie MB, Choi DW. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience 1995;68:615-619.


25. Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, et al. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke 2000;31:347-354.


26. Albers GW, Goldstein LB, Hall D, Lesko LM; Aptiganel Acute Stroke Investigators. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA 2001;286:2673-2682.


27. Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol 2002;1:383-386.


28. Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet 2020;395:878-887.

29. Park UJ, Lee YA, Won SM, Lee JH, Kang SH, Springer JE, et al. Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol 2011;121:459-473.


30. Hill MD. Efficacy and safety of nerinetide in participants with acute ischemic stroke undergoing endovascular thrombectomy excluding thrombolysis (ESCAPE-NEXT) [Internet]. Bethesda, MD: U.S. National Library of Medicine; 2020. [cited 2022 Jul 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT04462536.








