Introduction
Nonvalvular atrial fibrillation (NVAF) is a well-known risk factor for stroke, which is associated with cardiovascular mortality and morbidity. NVAF was associated with a five-fold increase in the incidence of ischemic stroke in the Framingham study [1]. Subsequent randomized clinical trials and meta-analyses showed that the use of an adjusted dose of vitamin K antagonist was associated with a 62% relative risk reduction in stroke [2]. Recently, considering the reduced risk of bleeding, direct oral anticoagulants have been accepted as the gold standard for preventing thromboembolic events in NVAF [3].
Most ischemic strokes in patients with NVAF are caused by cardiac embolism. In particular, left atrial or left atrial appendage (LA/LAA) thrombus can be noted in 10% of patients with atrial fibrillation and in more than 40% of patients with acute thromboembolism and newly recognized atrial fibrillation [4].
Despite several studies showing an increased occurrence of stroke in patients with LA/LAA thrombus, the prognostic implications remain unknown after stroke [5,6]. Considering the conditions prone to forming LA/LAA thrombi, patients may experience additional thromboembolic events or neurological worsening during admission. Embolism from a larger LA/LAA thrombus may be more organized due to its advanced age, which may be associated with worse recovery after stroke [7,8]. In this context, this study aimed to assess the effect of LA/LAA thrombi on the outcome of patients with ischemic stroke.
Methods
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Patients
This retrospective study used a prospective registry from a single tertiary center. Between January 2012 and December 2020, 6,489 consecutive patients with acute cerebral infarction or transient ischemic attack (TIA) within 7 days of stroke onset were admitted to the Severance Stroke Center and prospectively registered in the Specialized Multi-center Attributed Registry of sTroke (SMART) Registry. During admission, all patients were evaluated and managed using a standardized protocol that included computed tomography, magnetic resonance imaging, carotid Doppler, transcranial Doppler, 12-lead electrocardiography (ECG), and standard blood tests. Cardiac embolic sources were evaluated using transthoracic echocardiography, transesophageal echocardiography (TEE), and 24-h Holter monitoring. Additionally, a continuous ECG was performed during the stroke unit stay. This study was approved by the Institutional Review Board of Yonsei University College of Medicine (approval number: 4-2022-0518), and the requirement for informed consent was waived owing to the retrospective nature of the study.
LA/LAA thrombus
To determine the presence of potential sources of cardiac embolism, including LA/LAA thrombi, an echocardiographic study was routinely performed during admission. At our stroke center, TEE was also a part of the routine examination unless it could not be performed because of either the patient’s condition or failure to obtain informed consent. The TEE was performed by an experienced cardiologist. The TEE machine used a multiplane 5 MHz transducer attached to the tip of a gastroscope. Local pharyngeal anesthesia with 10% topical lidocaine was administered before the start of the examination. Transgastric, midesophageal, and basal views were recorded in a motion picture format. The presence of an intracardiac mass inside the left atrium and the left atrium appendage was also assessed. The Valsalva maneuver was performed with intravenous agitated saline injection to assess the presence of a right-to-left shunt.
In addition, between July 2006 and January 2017, multi-detector coronary computed tomography (MDCT) was consecutively performed for patients meeting any one of the following criteria [9] : (1) presence of atherosclerosis in cerebral arteries; (2) presence of two or more risk factors for coronary artery disease, such as hypertension, diabetes mellitus, dyslipidemia, smoking, and central obesity; and (3) men aged over 45 years and women aged over 55 years. MDCT was not performed when (1) high pulse rates were not controlled with beta-blockers at the time scheduled for MDCT (pulse rate >65 beats per minute), (2) the patient could not tolerate the study, (3) renal function was impaired with an estimated globular filtration rate of <60 mL/min/1.73 m2, or (4) informed consent could not be obtained. Previously, MDCT was reported to have a sufficiently high detection rate to identify various cardioembolic sources, including LA/LAA thrombi [10].
Clinical and echocardiographic variables
We collected data on vascular risk factors, such as hypertension, diabetes, dyslipidemia, coronary artery occlusive disease, congestive heart failure, and atrial fibrillation type (paroxysmal or persistent/permanent). Atrial fibrillation was defined as paroxysmal when arrhythmia spontaneously resolved within 7 days. Stroke severity was determined using the National Institutes of Health Stroke Scale (NIHSS), whereas functional status was assessed using the modified Rankin Scale (mRS). Using TEE with or without MDCT, the ejection fraction, early mitral inflow velocity to mitral annular early diastolic velocity (E/e’) ratio, and presence of aortic atheroma were determined for each patient. Aortic atheroma was defined as the presence of atherosclerosis in the aortic arch or ascending aorta. The presence of relevant arterial occlusion was assessed using magnetic resonance angiography and/or computed tomography angiography. Relevant arterial occlusion was defined as an occlusive arterial lesion supplying an acute-infarcted area. The reperfusion therapy included intravenous alteplase infusion and endovascular thrombectomy.
Outcome measures
The main outcome measured in this study was poor outcome, defined as an mRS score of >3 at 90 days after the index stroke event [11]. Furthermore, we also investigated the association between LA/LAA thrombus and outcomes defined as an mRS score of >1 or >2. In addition, we collected data on the occurrence of ischemic stroke (including TIA), hemorrhagic stroke (including intracerebral hemorrhage, intraventricular hemorrhage, subarachnoid hemorrhage, and subdural hemorrhage), myocardial infarction, bleeding events, major adverse cardiovascular events (MACE; a composite of stroke [ischemic or hemorrhagic], myocardial infarction, and cardiovascular death), and all-cause mortality during the 90-day follow-up period. Stroke or TIA was determined by a stroke specialist based on the clinical and radiological findings. Myocardial infarction was defined as the diagnosis of acute myocardial infarction based on clinical symptoms, cardiac enzymes, ECG, and imaging studies, such as coronary angiography. All bleeding events were recorded, including gastrointestinal, respiratory, and muscular bleeding requiring transfusion or hospitalization. Stroke neurologists and research nurses regularly contacted the patients or their caregivers during follow-up via regular face-to-face visits or telephone interviews, with or without medical chart review, to investigate outcomes, including mortality. In addition, we investigated the NIHSS score at discharge, occurrence of early neurological deterioration (END) during hospitalization, and duration (days) of hospital stay. END was defined as worsening of the total NIHSS score by more than 2 or any worsening of the NIHSS subscale on motor function of the arms or legs during admission. When patients with END underwent brain magnetic resonance imaging, we determined the infarction growth or the presence of new ischemic lesions using brain diffusion-weighted imaging performed at admission and at the time of END.
Statistical analysis
Descriptive statistics for the variables included in this study were analyzed. Categorical values are reported as counts with proportions and continuous variables as medians with interquartile ranges (IQRs). A comparative statistical analysis was performed between patients with and without LA/LAA thrombi. The chi-square test was performed for categorical variables and the Mann-Whitney U test was performed for continuous variables. Multivariable logistic or ordinal regression analysis was performed to assess the impact of LA/LAA thrombi on outcomes after eliminating the effect of covariates. Cox proportional hazards regression analysis was used to compare the events that occurred during follow-up. Data for the E/e’ ratio (48 patients, 7.6%) and ejection fraction (10 patients, 1.6%) were missing. Missing data were imputed using predictive mean-matching.
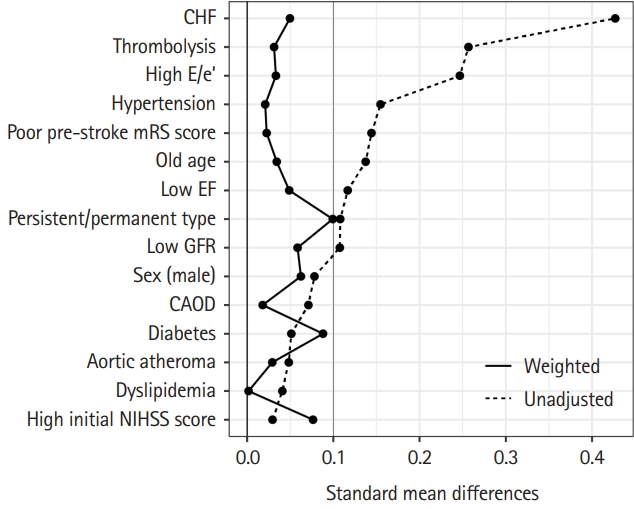
Furthermore, to reduce the potential effects of selection bias and confounding factors, we estimated the propensity scores for all patients enrolled for performing inverse probability of treatment weighting in this retrospective cohort study. Propensity scores were calculated for each patient using a logistic regression model including predetermined variables such as age (>75 years), sex, history of hypertension, diabetes, coronary artery occlusive disease, dyslipidemia, congestive heart failure, premorbid disability (mRS >3), glomerular filtration rate (>30 mL/min/1.73 m2), ejection fraction (>40%), E/e’ ratio (>15), presence of aortic atheroma, persistent/permanent atrial fibrillation, initial NIHSS score (>10), treatment with intravenous alteplase injection, and endovascular thrombectomy. The nearest neighborhood method was used, with a ratio of 1:2. Inverse probability of treatment weighting was calculated by using an inverse propensity score [12]. Symmetrical trimming was performed with a delta of 0.067, which is a previously proposed optimal cutoff, to achieve the final weighted dataset [13]. Balance was assessed using the standardized mean difference between the two groups before and after weighting. A 10% cutoff was used to define a significant imbalance [14]. P-values were two-sided. Multivariable analysis was performed to obtain the odds ratio for the weighted dataset. All statistical analyses were performed using R 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria) [15].
Results
Patients
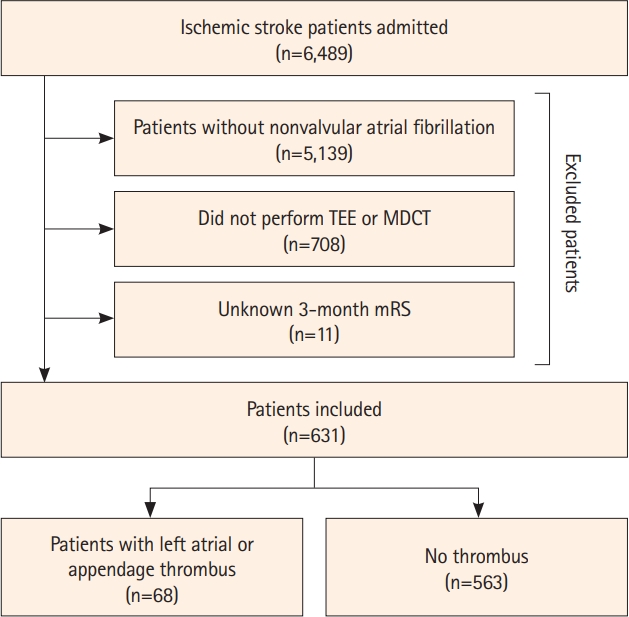
Of the 6,489 patients admitted during the study period, we excluded 5,139 (79.2%) without NVAF, 708 (10.9%) who did not undergo TEE or MDCT, and 11 (0.2%) who were not followed up. Finally, 631 patients (9.7%) were included in this study (Figure 1). The median age of the included patients was 78.0 (IQR 70.0 to 83.0) years, and 57.7% of the patients were males. Compared with the patients excluded from this study, those included were older and more likely to have a history of hypertension, coronary artery occlusive disease, congestive heart failure, aortic atheroma, and worse glomerular filtration rate, and had a higher initial NIHSS score (Supplementary Table 1).
There were 68 (10.7%) patients with LA/LAA thrombus (56 observed on TEE and 26 observed on MDCT). Patients with LA/LAA thrombus were more likely to have congestive heart failure and persistent/permanent atrial fibrillation (22.1% vs. 5.7% and 95.6% vs. 73.5%, standardized mean difference 42.7% and 10.8%, respectively) (Table 1) than those without LA/LAA thrombus. Upon discharge, patients with LA/LAA thrombus were significantly more likely to be prescribed with a vitamin K antagonist than those without thrombus (75.0% vs. 43.2%, P<0.001).
Comparison of the 3-month outcome
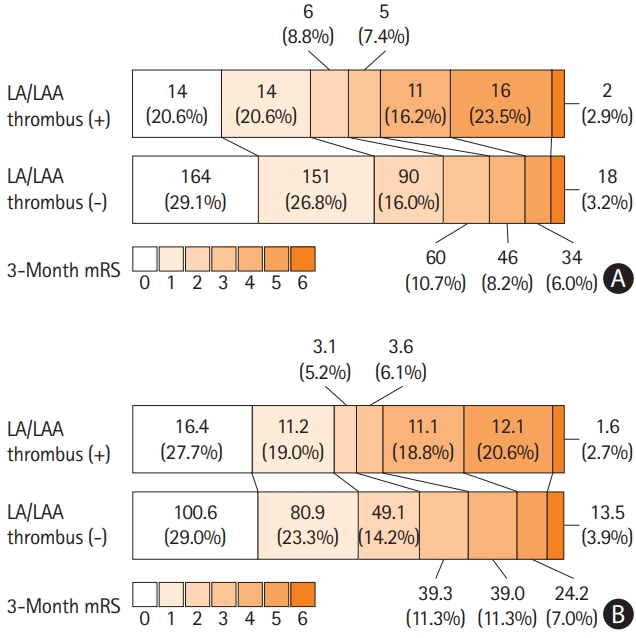
The distribution of 3-month mRS scores is shown in Figure 2. Patients were likely to have a poor outcome when an LA/LAA thrombus was observed (42.6% vs. 17.4%, P<0.001) (Table 2). In the univariable analysis, a poor outcome was associated with the presence of LA/LAA thrombus along with age, male sex, history of diabetes, coronary artery occlusive disease, worse glomerular filtration rate, premorbid disability, high initial NIHSS score, and E/e’ ratio (>15) (all P<0.05). Multivariable logistic regression analysis adjusted for these significant factors on univariable analysis showed that LA/LAA thrombus was an independent determinant of a poor outcome (odds ratio [OR] 3.73, 95% confidence interval [CI] 2.03-6.83, P<0.001) (Supplementary Table 2).
In addition, inverse probability of treatment weighting analysis resulted in effective sample sizes of 59.0 for the group with LA/LAA thrombus, and 346.5 for the group without LA/LAA thrombus (Table 1). All the characteristics of the two groups were wellbalanced, as the standardized mean differences were not significant for any of the variables (Table 1 and Figure 3). Inverse probability of treatment weighting analysis demonstrated that LA/LAA thrombus was also independently and significantly associated with poor outcome (OR 4.05, 95% CI 1.98-8.32, P<0.001) (Table 3). LA/LAA thrombus was also an independent determinant when poor outcome was defined as mRS >2 (OR 2.55, 95% CI 1.31-4.99, P=0.006) but not on poor outcome with mRS >1 (OR 1.53, 95% CI 0.81-2.88, P=0.194) (Table 3). Multivariable ordinal regression analysis showed that LA/LAA thrombus was a significant factor for a shift in mRS score at 90 days (OR 1.69, 95% CI 1.01-2.83, P=0.046) (Supplementary Table 3). However, Cox proportional hazards regression analysis showed that LA/LAA thrombus was not associated with the occurrence of ischemic stroke, bleeding events, MACE, or all-cause mortality, while the risk of hemorrhagic stroke and myocardial infarction was different in the presence of LA/LAA thrombus (Supplementary Table 4).
Comparison of short-term prognosis
When we compared the occurrence of END, NIHSS score at discharge, and relevant arterial occlusion between patients with and without LA/LAA thrombus (Table 2), patients with LA/LAA thrombus were more likely to have relevant arterial occlusive lesions than those without LA/LAA thrombus (32.4% vs. 20.6%, P=0.040 in the original population and 36.3% vs. 22.4%, P=0.047 in the inverse probability of treatment weighting population). For patients with relevant artery occlusion, carotid artery occlusion was more common in those with LA/LAA thrombus (Supplementary Table 5). Patients with relevant arterial occlusive lesions were more likely to have higher median NIHSS scores at stroke presentation than those without these lesions (median [IQR]: 8 [3-16] vs. 3 [1-8], P<0.001 in the original population and 11 [3-16] vs. 3 [1-8], P<0.001 in the inverse probability of treatment weighting population). In addition, the median NIHSS score at discharge was higher in patients with LA/LAA thrombus in the original population (2 [0-6.5] vs. 1 [0-3], P=0.013), and the median hospital stay (days) was longer before and after adjustment in patients with LA/LAA thrombus (8.5 [7-12] vs. 7 [5-9], P<0.001 in the original population and 8 [6.5-10] vs. 7 [5-9.5], P<0.001 in the inverse probability of treatment weighting population) than in those without LA/LAA thrombus. Among 59 patients with END, 36 underwent repeated brain diffusion-weighted imaging, and 50.8% had infarction growth. However, in terms of END during hospitalization, infarction growth, or MACE within 3 months, there was no significant difference between the two groups. When we investigated whether there was any difference in the outcomes for patients with LA/LAA thrombus after discharge according to the type of oral anticoagulant (vitamin K antagonists vs. direct oral anticoagulants), there was no difference between the groups (Supplementary Table 6).
Discussion
This study showed that the presence of LA/LAA thrombus was associated with a worse 3-month mRS score when detected during the evaluation for acute ischemic stroke. Multivariable and inverse probability of treatment weighting analyses consistently showed an independent and significant association between LA/LAA thrombus and poor functional outcome 3 months after stroke. These relationships can be attributed to persistent relevant arterial occlusion and a higher NIHSS score at discharge rather than a higher risk of early recurrent stroke or MACE within 3 months after stroke.
LA/LAA thrombus was detected in 10.7% of our stroke patients with atrial fibrillation, similar to the results of previous studies (weighted mean of 9.8% in a previous meta-analysis) [4]. The prevalence of LA/LAA thrombus might be dependent on the patients’ comorbidities, modality for evaluation of the thrombus, and anticoagulation therapy. Although TEE is the gold standard diagnostic method for detecting LA/LAA thrombus, showing 100% sensitivity and 99% specificity compared with results from cardiac surgery [16], TEE can be invasive and requires patient cooperation and thus may be difficult to perform during the acute stroke period [5,17]. In our study, we also used MDCT, which is a part of the routine evaluation for patients at our stroke center. MDCT is a noninvasive but accurate screening process for detecting intracardiac thrombi that may lead to reliable detection of LA/LAA thrombi [18,19].
In our study, the presence of an LA/LAA thrombus was associated with congestive heart failure and persistent/permanent atrial fibrillation. Thrombus formation within the heart can be influenced by abnormal changes in vessel wall, flow, and blood constituents [20]. Previous reports have demonstrated that LA/LAA thrombi may be associated with advanced age, female sex, hypertension, diabetes, a high CHA2DS2-VASc score, and low ejection fraction or congestive heart failure [4,21-23]. These conditions could be related to an enlarged left atrium or prolonged stasis in the left atrium, along with a more thrombogenic hemostatic milieu within the heart. Persistent or permanent atrial fibrillation may carry a high thromboembolic risk [24]; therefore, the burden of atrial fibrillation can affect progressive structural changes in the left atrium, subsequently leading to increased risk to thrombus formation within the heart [25], as shown in our results.
Patients with LA/LAA thrombus could have an approximately three times higher risk of poor outcome 3 months after ischemic events, probably owing to index stroke severity, END after stroke, recurrent ischemic stroke, or MACE within 3 months. In our study, there were no differences in the frequency of initial stroke severity, END, or MACE between patients with and without LA/LAA thrombi. However, patients with LA/LAA thrombus were more likely to have relevant arterial occlusion and longer hospital stay than those without LA/LAA thrombus. Although stroke severity at presentation was similar between patients with and without LA/LAA thrombus, a higher frequency of relevant arterial occlusion in the former implied a more organized thrombus resistant to endogenous thrombolytic activity. In addition, patients with LA/LAA thrombus were more likely to have proximal artery occlusion, which may imply that LA/LAA thrombus was related to the larger size of the thrombi. These results indicate a high likelihood of infarction growth and slow recovery during the acute post-stroke period. Our findings indicate that the high probability of worse outcomes in patients with LA/LAA thrombus was mainly attributed to the index stroke prognosis rather than to a recurrent embolic event or MACE. However, further studies are needed to understand the reason behind the poor prognosis of patients with LA/LAA thrombi.
To manage LA/LAA thrombi, current guidelines recommend vitamin K antagonists as the drug of choice [26]. In our study, patients with LA/LAA thrombus were more likely to receive vitamin K antagonists than direct oral anticoagulants. However, there was no difference in outcomes, such as MACE or recurrent stroke, according to the type of oral anticoagulant used at discharge. In addition, the distribution of the 3-month mRS score did not differ between patients with LA/LAA thrombi who were prescribed direct oral anticoagulants and vitamin K antagonists. Considering that direct oral anticoagulants may be a convenient and effective alternative to vitamin K antagonists, the use of direct oral anticoagulants for managing LA/LAA thrombi could be worthy of further investigation [27]. There is scarce evidence on aggressive intervention, including endovascular or open surgery, for cases of failure with medical management. Increasing the international normalized ratio target or adding or switching to low-molecular-weight heparin may be considered for patients with residual thrombus after adequate medical treatment [28].
This study has several limitations. This study was retrospective and could have been subject to a selection bias. Although multivariable analysis adjusted for potential confounders and inverse probability of treatment weighting was used to minimize the bias caused by the retrospective nature of the study, there might have been other confounding variables that were not measured in this study. Moreover, although many patients were excluded from this study because they did not undergo TEE, TEE was part of the standard examination at our center regardless of patient characteristics. Furthermore, various cardiologists performed TEE during the study period. The results may not be generalizable because there were significant differences in baseline characteristics between the included and excluded patients. Additionally, owing to the retrospective nature of the study, the medication compliance within 3 months after stroke was not assessed. The clinical implications of this study may be limited by the lack of evidence for additional aggressive management after LA/LAA thrombus detection.
Conclusions
Ischemic stroke patients with LA/LAA thrombus are at risk of worse functional outcomes after 3 months. The hospital stay was longer and relevant artery occlusion was more frequent in patients with LA/LAA thrombus than in those without LA/LAA thrombus. The prognostic implications of this study may be useful for predicting the clinical outcomes and management of acute stroke.