Venous Outflow Profiles Are Linked to Clinical Outcomes in Ischemic Stroke Patients with Extensive Baseline Infarct
Article information
Abstract
Background and Purpose
The benefit of endovascular thrombectomy (EVT) treatment is still unclear in stroke patients presenting with extensive baseline infarct. The use of additional imaging biomarkers could improve clinical outcome prediction and individualized EVT selection in this vulnerable cohort. We hypothesized that cerebral venous outflow (VO) may be associated with functional outcomes in patients with low Alberta Stroke Program Early CT Score (ASPECTS).
Methods
We conducted a retrospective multicenter cohort study of patients with acute ischemic stroke due to large vessel occlusion (AIS-LVO). Extensive baseline infarct was defined by an ASPECTS of ≤5 on admission computed tomography (CT). VO profiles were assessed on admission CT angiography using the Cortical Vein Opacification Score (COVES). Favorable VO was defined as COVES ≥3. Multivariable logistic regression was used to determine the association between cerebral VO and good clinical outcomes (90-day modified Rankin Scale score of ≤3).
Results
A total of 98 patients met the inclusion criteria. Patients with extensive baseline infarct and favorable VO achieved significantly more often good clinical outcomes compared to patients with unfavorable VO (45.5% vs. 10.5%, P<0.001). Higher COVES were strongly associated with good clinical outcomes (odds ratio, 2.17; 95% confidence interval, 1.15 to 4.57; P=0.024), independent of ASPECTS, National Institutes of Health Stroke Scale, and success of EVT.
Conclusions
Cerebral VO profiles are associated with good clinical outcomes in AIS-LVO patients with extensive baseline infarct. VO profiles could serve as a useful additional imaging biomarker for treatment selection and outcome prediction in low ASPECTS patients.
Introduction
Landmark randomized clinical trials have demonstrated the strong efficacy of endovascular thrombectomy (EVT) treatment in patients with acute ischemic stroke due to large vessel occlusion (AIS-LVO) [1-5]. However, these trials only included a highly selected group of patients with small baseline infarct sizes, and largely excluded patients with extended ischemic core lesions, commonly defined by an Alberta Stroke Program Early CT Score (ASPECTS) of ≤5 [6,7]. Most recently, the Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism Japan Large IscheMIc core Trial (RESCUE-Japan LIMIT) provided first high-level evidence of EVT benefit in patients with a low ASPECTS [7], who represent about 25% of all AIS-LVO patients [8]. Five other randomized controlled trials are currently enrolling patients to address this persistent challenge in EVT [9]. While awaiting their results with great interest, the identification of additional prognostic parameters might be crucial for clinical outcome prediction and, potentially, EVT selection in patients with extensive baseline infarct.
Arterial collaterals have been extensively studied in stroke research which, if present, may sustain salvageable brain tissue and reduce ischemic tissue damage [10-14]. In low ASPECTS patients, successful EVT was linked to improved clinical outcomes in dependence of good arterial collaterals [15]. However, cerebral perfusion is not exclusively determined by its arterial inflow, but also by its venous outflow (VO) [16]. Insufficient inflow by upstream arterial collaterals, together with compromised blood transit through ischemic tissue, may result in a poor venous drainage of ischemic brain tissue [17]. Cerebral VO profiles can be assessed using the Cortical Vein Opacification Score (COVES) on computed tomography angiography (CTA) without the need for additional imaging [18]. Recent studies highlighted a potential major role of cortical VO profiles in cerebral microvascular perfusion assessment, EVT success, and ischemic edema formation [16,17,19,20].
To date, it has not been investigated whether VO profiles also constitute an independent prognostic marker in low ASPECTS patients. We hypothesized that more robust VO profiles are associated with better clinical outcomes and may serve as an additional selection criterion for EVT treatment in AIS-LVO patients presenting with extensive baseline infarct (ASPECTS of ≤5).
Methods
Study design
In this retrospective cohort study, we analyzed the continuously maintained databases of two high-volume stroke centers between October 2013 and January 2021. We enrolled patients with acute ischemic stroke meeting the following inclusion criteria: (1) EVT triage within 16 hours after symptom onset; (2) pretreatment non-contrast computed tomography (NCCT) of the head and single-phase CTA with sufficient opacification of intracranial venous vessels to determine cortical VO profiles as described in previous studies [16,17]; (3) acute ischemic stroke due to anterior circulation large vessel occlusion of the internal carotid artery or of the M1 or M2 segment of the middle cerebral artery (MCA); (4) extensive baseline stroke defined as ASPECTS of ≤5 on pretreatment NCCT; and (5) documentation of 90-day modified Rankin Scale (mRS) scores by a stroke neurologist or registered study nurse. Baseline patient characteristics were extracted from the electronical medical records. Patients were dichotomized into good clinical outcomes (GCOs; 90-day mRS score of 0–3) and poor clinical outcomes (PCOs; 90-day mRS score of 4–6) based on previous studies [7,15,21,22]. Excellent mechanical recanalization status was defined as thrombolysis in cerebral infarction (TICI) scores of 2c or 3 and good vessel recanalization was defined as TICI grade of 2b, 2c, or 3 on digital subtraction angiography images.
The study was approved by the Institutional Review Boards at each of the included centers (University Medical Center Hamburg-Eppendorf and Stanford University School of Medicine). The study was conducted in accordance with the ethical guidelines of the local ethics committee and in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). Informed consent was waived by our Institutional Review Boards due to the retrospective design of the study.
Image analysis
ASPECTS ratings were used to determine the extent of early cerebral ischemic infarction on pretreatment NCCT [23,24]. The rating is based on a binary assessment of 10 predefined regions of interest within the MCA territory for hypoattenuation of brain parenchyma. Lower ASPECTS values indicate larger ischemic infarction. Extensive baseline infarct was assumed when an ASPECTS of ≤5 was obtained [9]. ASPECTS ratings were performed by two experienced neuroradiologists (T.D.F. and J.J.H., with 10 and 15 years of working experience). Discrepancies were settled by consensus readings.
VO profiles were assessed on admission single-phase CTA by determining the COVES, which reflects the major VO pathways of the MCA territory [18]. COVES ranges from 0 to 6 points. The score is obtained by grading the opacification of three major cortical veins, namely the superficial middle cerebral vein, vein of Labbé, and sphenoparietal sinus, on a scale from 0 to 2 points (0, not visible; 1, moderate opacification; 2, full opacification). A favorable VO was defined as COVES ≥3 based on previous studies [17,20]. VO grading was independently performed by two neuroradiologists (T.D.F. and J.J.H., with 10 and 15 years of working experience) with substantial inter-reader agreement [17]. Only patients with a sufficient CTA imaging quality (complete opacification of the dural and sigmoid sinuses) were included [16,17].
Arterial collateral status was assessed on CTA images based on the Maas scale [12]. The Maas scale is a 5-point score from 1 to 5 points which grades the extent of collateral vessels in the sylvian fissure and leptomeningeal convexity, reflecting the MCA territory. A favorable Maas score was defined as an equal or greater arterial filling of the affected hemisphere compared to the unaffected hemisphere (Maas score ≥3). Ratings were performed by two experienced neuroradiologists (T.D.F. and J.J.H., with 10 and 15 years of working experience).
A fully automated software (RAPID, iSchemaView, Menlo Park, CA, USA) was used to analyze computed tomography (CT) perfusion studies. Baseline ischemic core volume was identified by RAPID as tissue with a relative cerebral blood flow (CBF) of <30% compared to mean CBF of the contralateral unaffected hemisphere.
Primary outcome measure
The 90-day mRS scores were used as primary outcome measure. GCO was defined as 90-day mRS score of 0–3 based on previous studies of low ASPECTS patients [7,15,21,22].
Statistical analysis
Statistical analyses and data visualization were conducted using R statistical software version 4.1.2 (R Project for Statistical Computing, Vienna, Austria) and RStudio statistical software version 2021.09.1+372 (Rstudio). Patient characteristics, imaging data and treatment outcomes were compared between patients with GCO (90-day mRS score of 0–3) and PCO (90-day mRS score of 4–6). Categorical variables were reported as counts and percentages. Continuous variables were reported as median and interquartile range. Categorical variables and continuous variables were compared using chi-squared test and Mann-Whitney U test, respectively. A multivariable logistic regression model was designed to determine factors independently associated with the primary outcome (GCO). Variables with a P≤0.10 in the univariate analysis (Tables 1 and 2) were included as independent variables into the main regression model, namely age, sex, admission National Institutes of Health Stroke Scale (NIHSS), ASPECTS, COVES, Maas score, administration of intravenous lysis using tissue plasminogen activator (tPA) and excellent mechanical recanalization (Table 3). A second logistic regression model was set up replacing excellent mechanical recanalization (TICI 2c/3) with good mechanical recanalization (TICI 2b/2c/3) (Supplementary Table 1). We calculated the variance of inflation factor (VIF) for each independent variable to exclude considerable multicollinearity in the regression models. VIF values did not indicate critical multicollinearity (VIF values were consistently <3) [25]. Moreover, we determined Akaike Information Criterion (AIC) values as a relative measure of model quality for each regression. Note that AIC improved by adding VO as an independent variable to the main model (compare Table 3 and Supplementary Table 2). A twotailed P<0.05 was considered statistically significant for all conducted analyses.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Baseline patient characteristics
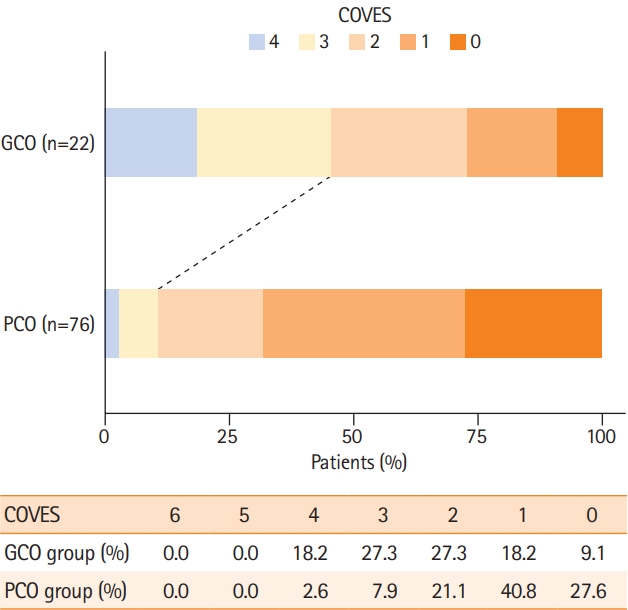
A total of 98 patients who underwent EVT triage with extensive baseline infarcts (ASPECTS ≤5) met the inclusion criteria (Supplementary Figure 1). Among these patients, the median age was 77 years (interquartile range, 64 to 82), 56 (57.1%) were women and the median ASPECTS was 5 (range, 0 to 5 points) (Table 1). On pretreatment NCCT, 86 (87.8%) patients demonstrated an ASPECTS of 3–5 and 12 (12.2%) patients presented with an ASPECTS of 0–2. Favorable arterial collaterals were observed in 34 (34.7%) patients and therefore almost two times as frequent as favorable VO profiles (COVES ≥3) which were present in 18 (18.4%) patients only (Figure 1). It should be noted that in this low ASPECTS cohort, the highest observed COVES was 4 out of 6 possible points. Fifty-two (53.1%) patients received intravenous tPA and 88 (89.8%) patients underwent subsequent EVT (Table 2). On 90-day follow-up, 22 (22.4%) patients achieved GCO (defined as 90-day mRS score of 0–3), while 76 (77.6%) patients exhibited PCO (defined as 90-day mRS score of 4–6). For further comparison, we divided the low ASPECTS patients into two groups according to their 90-day mRS scores (GCO group and PCO group, respectively).

Patient with acute ischemic stroke due to occlusion of the right internal carotid artery presenting with favorable cerebral venous outflow profile. Green arrows indicate moderate/full venous contrast filling of the (A) vein of Labbé, (B) superficial middle cerebral vein, and (C) sinus sphenoparietalis. Red arrow indicates missing venous contrast filling.
Comparison between patients with good and poor clinical outcomes
GCO patients had a median 90-day mRS score of 2, and PCO patients had a median mRS score of 5 (P<0.001). The 24-hour NIHSS scores were also significantly lower in the GCO compared to the PCO group (median, 6 vs. 19, P<0.001). GCO patients were younger (median age, 65 years vs. 77 years, P=0.010), were more often men (14 of 22 [63.6%] vs. 28 of 76 [36.8%], P=0.025), had lower admission NIHSS (median, 12 vs. 19, P<0.001) and higher initial ASPECTS (median, 5 points vs. 4 points, P=0.002). GCO patients were more likely to exhibit favorable arterial collaterals (12 of 22 [54.5%] vs. 22 of 76 [28.9%], P=0.026). Both, location of arterial vessel occlusion (P=0.745) and time from symptom onset to imaging (median, 257 minutes vs. 210 minutes, P=0.343) did not significantly differ between the two groups. Notably, patients with GCO achieved an excellent mechanical recanalization status (TICI 2c/3) more frequently (13 of 20 [65.0%] vs. 25 of 68 [36.8%], P=0.025) compared to PCO patients, while there was no such difference for a good recanalization status (TICI 2b/2c/3) between both groups (17 of 20 [85.0%] vs. 47 of 68 [69.1%], P=0.161).
We investigated whether cortical VO profiles differed between the GCO and PCO group. VO profiles were more robust in GCO compared to PCO patients (median COVES of 2 point vs. 1 point, P<0.001). In total, 45.5% of GCO patients, but only 10.5% of PCO patients demonstrated favorable VO profiles on CTA (P<0.001). In presence of favorable VO profiles (COVES ≥3), favorable arterial collaterals were found in 66.7% of patients. The distribution of cortical VO profiles with respect to clinical outcomes are displayed in Figure 2.
Very low ASPECTS strongly predicts poor clinical outcome
We performed a sub-analysis at finer granularity to investigate the association between point-by-point ASPECTS, cortical VO and clinical outcomes in patients with extensive baseline stroke (Table 1 and Figure 3). The majority of GCO patients (77.3%) presented with an ASPECTS of 5 on pretreatment NCCT. Only a small number of GCO patients (22.7%) exhibited an ASPECTS of 3–4. Among the 86 patients with an ASPECTS of 3–5, favorable VO was observed in 17 (19.8%) patients. Of those patients with ASPECTS 3–5 and favorable VO, more than one out of two patients (58.8%) achieved GCO on 90-day follow-up (dark green stream fields in Figure 3), which was almost three times as many as in the entire cohort (22.4%). Among the 12 patients with a very low ASPECTS of 0–2, none achieved GCO on 90-day follow-up (dark red stream fields in Figure 3). Only a single patient with a very low ASPECTS of 0–2 (8.3%) demonstrated favorable VO.
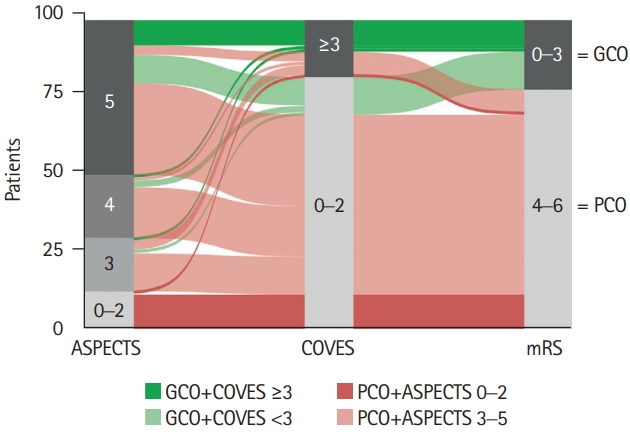
Alluvial diagram illustrating the clinical outcome (90-day modified Rankin Scale [mRS]) in relation to stroke extent on pretreatment non-contrast computed tomography (Alberta Stroke Program Early CT Score [ASPECTS]) and cortical venous outflow (Cortical Vein Opacification Score [COVES]). Green color spectrum for stream fields between blocks indicates good clinical outcome (GCO) and red color spectrum indicates poor clinical outcome (PCO). Note that no patient with an ASPECTS of 0 to 2 achieved GCO (dark red), while almost one in two with GCO exhibited favorable venous outflow (COVES ≥3; dark green).
Venous outflow is linked to good clinical outcomes in low ASPECTS patients
A multivariable logistic regression model was designed to determine factors that were independently associated with GCO in patients with an ASPECTS of ≤5. A total of 98 patients were included into the regression analysis. We found that higher COVES were strongly and independently associated with a favorable 90-day clinical outcome.
An 1-point increase in COVES, reflecting cerebral VO, increased the likelihood for GCO by the factor 2.17 (odds ratio [OR], 2.17; 95% confidence interval [CI], 1.15 to 4.57; P=0.024). Lower admission NIHSS (OR, 0.84; 95% CI, 0.72 to 0.96; P=0.017), higher ASPECTS (OR, 3.00; 95% CI, 1.30 to 9.24; P=0.025), and excellent mechanical recanalization defined as TICI 2c/3 (OR, 5.37; 95% CI, 1.33 to 25.82; P=0.024) were independently associated with greater odds of achieving GCO (Table 3), while good mechanical recanalization defined as TICI 2b/2c/3 did not (separate logistic regression model in Supplementary Table 1). Model estimates were adjusted for age, sex, arterial collateral status, and intravenous tPA administration. Favorable arterial collateral status did not reach statistical significance (P=0.902). There was no evidence for critical multicollinearity between arterial collateral status and VO (VIFMaas=1.57 and VIFCOVES=1.28). Predicted probabilities from the main logistic regression model in Table 3 are illustrated in Figure 4, showing the impact of VO, ASPECTS and excellent mechanical recanalization on clinical outcomes. After adjusting for baseline ischemic core volume instead of for ASPECTS, admission NIHSS, COVES, and excellent mechanical recanalization remained independent determinants of GCO (see separate logistic regression model in Supplementary Table 3).
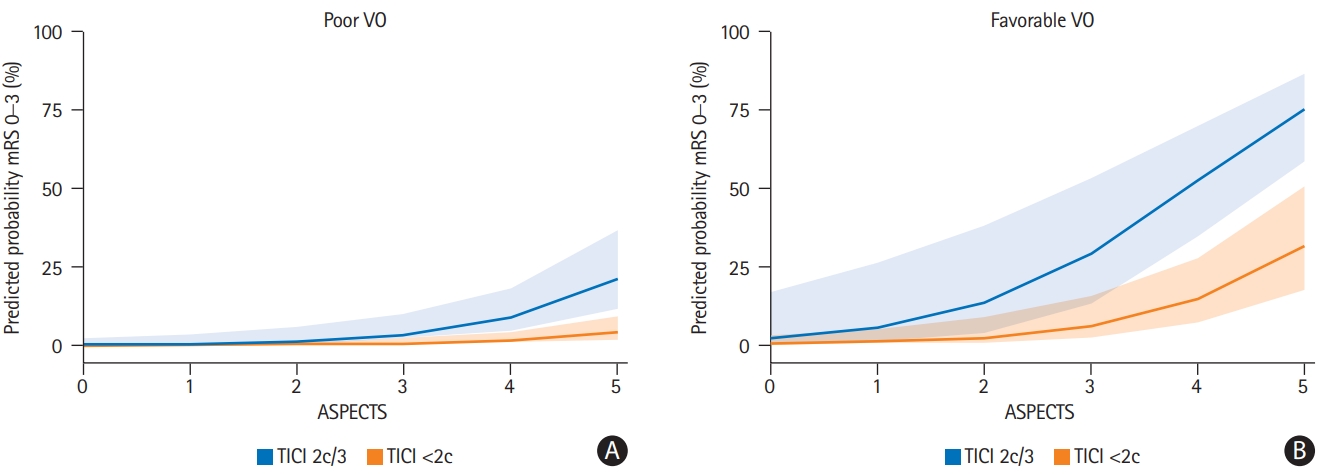
Predicted probabilities from the multivariable logistic regression model displayed in Table 3. Note that more favorable venous outflow (VO), higher Alberta Stroke Program Early CT Score (ASPECTS) and excellent mechanical recanalization thrombolysis in cerebral infarction (TICI) 2c/3 clearly increased the predicted probability of good clinical outcome (90-day modified Rankin Scale [mRS] of 0–3). Cortical Vein Opacification Score (COVES) was kept constant at 0 and 3 for (A) poor VO and (B) favorable VO, respectively. Admission National Institutes of Health Stroke Scale (NIHSS) was kept constant at 18 (median admission NIHSS). Shaded error bars represent one standard deviation.
We conducted a receiver operating characteristic (ROC) analysis to identify the “optimal” cutpoint for COVES to predict GCOs in low ASPECTS patients (Supplementary Figure 2). The analysis revealed an “optimal” cutpoint value of COVES ≥2 (95% CI, COVES ≥2–3), which is in the range of previous analyses [16,17].
Discussion
In this study, we investigated the impact of cerebral VO profiles on clinical outcomes in AIS-LVO patients with extensive baseline infarct on admission. To our knowledge, this is the first study which specifically analyzed cerebral VO and its prognostic value in a low ASPECTS cohort.
In total, we observed GCO in 22.4% of patients with extensive baseline infarct, which was in line with previous studies [15,21]. Favorable venous drainage was observed in only 18.4% of low ASPECTS patients and therefore notably less frequent than in previous studies which included patients with small ischemic core lesions (39.9%) [16]. Noteworthy, the GCO group presented with a much higher percentage of favorable VO profiles compared to the PCO group (45.5% vs. 10.5% of patients), suggesting that more robust venous drainage may serve as a valuable prognostic imaging biomarker in low ASPECTS patients. Accordingly, in logistic regression analysis, we found that VO was a strong and independent predictor of clinical outcomes in patients with extensive baseline stroke.
These findings can be explained by the interplay between cortical venous drainage, tissue microperfusion and cerebral edema formation. Tissue microperfusion and edema formation are significantly correlated in AIS-LVO [26]. At a pathophysiological level, cerebral tissue microperfusion via arteries and arterioles may be significantly hampered due to elevated interstitial pressure and increased vascular resistance in ischemic brain parenchyma [27,28]. Higher VO may indicate superior tissue microperfusion, as it reflects an approximation of the blood flow finally permeating the ischemic brain parenchyma. Furthermore, favorable VO profiles have been found to correlate strongly with reduced ischemic edema formation on admission and after successful reperfusion treatment [16,29]. Since both tissue microperfusion and edema formation are strong predictors of functional outcome [12,13], it is reasonable to assume that VO likewise may impact functional outcomes―also in extensive baseline strokes, as demonstrated by our study. Recent publications underline the clinical relevance of this complex collateral cascade, indicating that quantitative lesion water uptake might serve as a potential tool for EVT selection in low ASPECTS patients [16,30]. In comparison to tissue microperfusion assessment based on CT perfusion and quantitative measurement of cerebral edema, the advantage of cortical VO determination lies in its wide and rapid availability requiring only CTA with no need for additional imaging.
Our results indicate that favorable arterial collaterals did not significantly lower 90-day mRS after adjustment for VO profiles. Two out of three patients with favorable VO also demonstrated good arterial collateral flow. Therefore, it should be noted that the great overlap between favorable VO and good arterial collaterals make the statistical separation of their respective impact on clinical outcome more difficult. However, the lack of significant correlation between arterial collateral status and functional outcomes adds to the findings of previous studies [31], suggesting that collateral arterial blood flow to ischemic brain tissue may be impeded from transit through the tissue itself due to extensive edema formation [16,32]. Again, VO profiles could fill this gap and be more accurate in evaluation of beneficial residual microvascular perfusion in ischemic brain tissue. More studies are needed to investigate the underlying pathophysiological process of the uncoupling of arterial and venous collateralization. In addition, the group of patients exhibiting poor VO despite favorable arterial collateral flow (and vice versa) warrants additional study.
Notably, not a single patient achieved GCO when presenting with a very low ASPECTS of 0–2 on admission NCCT. Consistently, ASPECTS rating remained a strong and significant predictor of clinical outcome in the study cohort already preselected based on ASPECTS of 0–5. A sub-analysis of the Highly Effective Reperfusion Using Multiple Endovascular Stroke (HERMES) meta-analysis supports this observation, concluding that only patients with an ASPECTS of 3–5 might benefit from EVT [33]. Our data also revealed important differences among the lower ASPECTS scale and support a potential need for further subdivision of low ASPECTS patients, as already performed in ongoing randomized clinical trials, such as Efficacy and Safety of Thrombectomy in Stroke with Extended Lesion and Extended Time Window (TENSION) and Randomized Controlled trial to optimize patient’s selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT2) [6,34]. The recently published RESCUE-Japan LIMIT trial was also restricted to ASPECTS ratings of 3–5, arguing that patients with a very low ASPECTS of 0–2 are unlikely to regain functional independence simply due to the extent of unsalvageable infarct core [7].
We are currently awaiting further high-level evidence of efficacy and safety of EVT in patients with large ischemic cores. Our data suggests that excellent mechanical recanalization (TICI 2c/3) is associated with an improved clinical outcome in low ASPECTS patients, while good mechanical recanalization (TICI 2b/2c/3) did not achieve statistical significance. In general, the retrospective design of this study does not allow to draw definitive conclusions on EVT efficacy in extensive baseline strokes. Nonetheless, this finding falls in line with the results of the RESCUE-Japan LIMIT trial and retrospective studies, indicating a potential clinical benefit of EVT in low ASPECTS patients; here again, after a finer subdivision according to further patient characteristics and imaging biomarkers [7,15,30,33].
This study has some limitations. First, the retrospective and non-randomized study design may introduce selection bias. Prospective randomized controlled trials are needed to validate our findings and increase their generalizability. Second, the small number of patients, due to the strict inclusion and exclusion criteria for endovascular treatment, is a major limitation. However, one has to consider that endovascular treatment of low ASPECTS patients following current guidelines is recommended on an individual basis in selected cases [35], and is not standardized with widespread concerns about clinical equipoise. Third, venous contrast opacification depends, among others, on the amount of contrast media, the rate of contrast media injection, and its timing relative to image acquisition, as described previously [18]. We cannot systematically quantify the impact of these possible confounders on the COVES rating. However, to date the most important research works on VO profiles in ischemic stroke patients are based on single-phase CTA [16,18,19]. It requires further studies to investigate whether multi-phase CTA is significantly more appropriate than single-phase CTA for assessing cortical VO profiles. Fourth, there is relevant disagreement about how to define extensive ischemic lesion size on admission, which is also reflected by the different inclusion criteria of current randomized controlled trials enrolling low ASPECTS patients [6,34]. Of the six randomized controlled “large core” trials, only two trials use a combination of ASPECTS and perfusion imaging as selection strategy, while four trials defined large ischemic core size using ASPECTS alone [36,37]. In accordance with these trials, we defined the extent of the ischemic core using ASPECTS on admission NCCT, a wide and rapid applicable scoring system in a real-world scenario.
Conclusions
In AIS-LVO patients with extensive baseline infarcts (ASPECTS of ≤5) who underwent EVT triage, cerebral VO was strongly associated with good functional outcomes, independent of recanalization status, admission NIHSS and ASPECTS. This study highlights cerebral VO as a meaningful and widely available imaging biomarker for microvascular perfusion. VO assessment may offer a valuable additional imaging tool to guide treatment selection in low ASPECTS patients who most likely benefit from EVT. Future prospective studies should validate our findings.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.01046.
Multivariable binary logistic regression to predict good clinical outcome (mRS at 90-day follow-up 0–3) in patients with an ASPECTS ≤5 using good mechanical recanalization (TICI 2b/2c/3) as independent variable
Multivariable binary logistic regression to predict good clinical outcome (mRS at 90-day follow-up 0–3) in patients with an ASPECTS of ≤5 without COVES as independent variable
Multivariable binary logistic regression to predict good clinical outcome (mRS at 90-day follow-up 0–3) in patients with an ASPECTS of ≤5 using baseline ischemic core volume (CBF <30%) as independent variable
Flow chart with patient inclusion and exclusion criteria. EVT, endovascular thrombectomy; CTA, computed tomography angiography; ASPECTS, Alberta Stroke Program Early CT Score; NCCT, non-contrast computed tomography; ICA, internal carotid artery; MCA, medial cerebral artery; mRS, modified Rankin Scale; GCO, good clinical outcome; PCO, poor clinical outcome.
Estimation and validation of “optimal” cutpoint for cerebral venous outflow. (A) Distribution of Cortical Opacification Venous Score (COVES) values in low Alberta Stroke Program Early CT Score (ASPECTS) patients dichotomized by clinical outcomes. Good clinical outcome (GCO) was defined as 90-day modified Rankin Scale (mRS) score of 0–3 and poor clinical outcome (PCO) as 90-day mRS score of 4–6. Red vertical lines indicate the “optimal” cutpoint derived from receiver operating characteristic (ROC) analysis in (B). (B, C) ROC curve analysis (B) illustrating the diagnostic ability of COVES to discriminate between GCO and PCO in low ASPECTS patients (area under the curve [AUC], 0.78; sensitivity, 0.70; specificity, 0.76). Visualization of cutpoint variability using bootstrapping (n=1,000 bootstrap samples) in (C). Calculations revealed a threshold of COVES ≥2 as “optimal” cutpoint with a 95% confidence interval of COVES ≥2–3.
Notes
Disclosure
Gregory W. Albers reports equity and consulting for iSchemaView and consulting from Medtronic; Jens Fiehler reports grants and personal fees from Acandis, Cerenovus, MicroVention, Medtronic, Stryker, Phenox and grants from Route 92 outside the submitted work; Jeremy J. Heit reports consulting for Medtronic and MicroVention and Medical and Scientific Advisory Board membership for iSchemaView; Tobias D. Faizy reports grants from the German Research Foundation (DFG) during the conduct of the study.
Acknowledgements
Tobias Djamsched Faizy was funded by the German Research Foundation (DFG) for his work as a postdoctoral research scholar at Stanford University, Department of Neuroradiology (Project Number: 411621970); Max Wintermark reports grants and funding from the NIH under the grant numbers (1U01 NS086872-01, 1U01 NS087748-01, and 1R01 NS104094).