Elderly CADASIL patients with intact neurological status
Article information
Abstract
Background and Purpose
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is one of the most devastating cerebral small vessel diseases. However, despite its progression with aging, some patients remain neurologically intact (Nint) even when they get older. Their main characteristics are poorly known. We aimed to delineate their clinical, imaging, and molecular features.
Methods
Individuals aged over 65 years were selected from a cohort of 472 CADASIL patients. Subjects who had no focal deficit, cognitive impairment, or disability were considered Nint. Their demographic, genetic, clinical, and imaging features were compared to those with permanent neurological symptoms (Nps).
Results
Among 129 patients, 23 (17.8%) individuals were considered Nint. The frequency of vascular risk factors and NOTCH3 cysteine mutations in epidermal growth factor-like repeat (EGFr) domains 7-34 did not differ between Nint and Nps patients but Nint patients had less stroke events and were more likely to have migraine with aura. The number of lacunes and microbleeds and degree of brain atrophy were lower in the Nint group, but the volume of white matter hyperintensities did not differ between the two groups.
Conclusions
Nearly one in five CADASIL patients can remain Nint after the age of 65 years. Their clinical and imaging profile differed from that of other age-matched CADASIL patients. The location of NOTCH3 mutation inside or outside EGFr domains 1-6 cannot fully explain this discrepancy. The factors involved in their relative preservation of brain tissue from severe damage despite aging remain to be determined.
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common inherited cerebral small vessel disease (cSVD) [1]. Although the spectrum of clinical manifestations appears very broad and their severity highly varies over decades of disease progression, motor disability and cognitive decline develop inexorably in most older patients with a confirmed diagnosis of CADASIL. In one of the largest samples reported so far, more than 90% of patients aged more than 65 years already experienced one or more strokes and half were severely demented and bedridden [2]. The median life expectancy was estimated around 65 years in men and 71 years in women [2]. Similar findings have been reported in multiple large cohorts of symptomatic patients [3,4]. Thus, although its rate of clinical progression appears highly variable over decades, CADASIL is mainly considered to be one of the most devastating cSVDs that, ultimately, always converges to severe disability with increasing age [1].
In addition to age, different factors have been identified as potentially modulating the disease progression along aging. The ε2 allele of apolipoprotein E was found associated with a larger volume of white matter hyperintensities (WMHs) [5], male sex with a higher risk of cognitive decline and more lacunes and cerebral atrophy [6], smoking with earlier and more incident stroke events [7], higher blood pressure with an increased risk of cognitive decline [8]. More recently, after typical NOTCH3 mutations in domains 7-34 were discovered at an unexpectedly high frequency in the general population, the location of cysteine mutations in epidermal growth factor-like repeat (EGFr) domains 1-6 versus 7-34 was also shown to have a strong detrimental effect on this clinical variability [9]. Some elderly individuals having mutations in EGFr domains 7-34 were even found to present with very limited cerebral lesions [10]. This does not rule out that patients with variants located in EGFr domain 1-6 cannot remain neurologically intact (Nint) or stable [11].
In parallel, during the recent decade and with the spread of genetic testing, diagnosis of CADASIL has been broadened to include patients with more atypical symptoms or much less severe clinical picture. Moreover, the sensitivity of genetic testing in daily practice has been improved by allowing the search of cysteine mutations in all exons encoding for EGFr domains whereas the diagnostic test was initially limited to only few exons. Thus, the number of elderly patients diagnosed with the disease has increased, not only because of the aging of patients diagnosed many years ago but also because of the increased diagnostic rate in the elderly even in presence of transient or benign neurological manifestations or after incidental discovery of white matter signal changes.
Specific analysis of cases who remain neurologically normal despite the significant advancement in age may provide insight into the different sources of clinical variability in the presence of NOTCH3 gene mutations. The demographics, clinical, imaging, and genotype characteristics of this population remain however poorly understood. In the present study, we aimed to better delineate these features by comparing elderly CADASIL patients with intact neurological status to the other patients of identical age but with some permanent symptoms.
Methods
Patients
All patients over 65 years of age at their last follow-up visits were selected for the present study from a long-term prospective cohort of 472 CADASIL patients. These patients were recruited at the National Referral Center for Rare Cerebrovascular disorders in France (CERVCO, www.cervco.fr) from September 2003 up to November 2021. All of them harbored a typical cysteine mutation within the NOTCH3 gene and gave their written consent for collecting their biological, clinical, and imaging data according to a detailed predefined protocol [12] during their follow-up [13]. This study was approved by an independent ethics committee (updated agreement CEEI-IRB-17/388) and conducted in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice and General Data Protection Regulation (GDPR) in Europa.
Clinical parameters
All clinical and demographic data were collected at study entry and updated during follow-up. They included age, sex, years of education, and vascular risk factors. History of hypertension was defined as a previous diagnosis of hypertension (blood pressure >140/90 mm Hg) or use of antihypertensive treatment for control of blood pressure. Blood pressure was also measured at baseline and at follow-up visits. “Current smoking” was defined as active smoker at baseline, while “ever smoker” was defined as active smoker or past smoker. A history of transient ischemic attacks (TIAs) or stroke, and dementia defined according to Diagnostic and Statistical Manual of Mental Disorders (5th edition), were obtained at baseline and at follow-up visits. Subjects were regularly interviewed and evaluated for cognitive impairments. They also underwent a detailed neurological and neuropsychological examination at each follow-up visit. Any focal neurological deficit was systematically recorded, and the National Institutes of Health Stroke Scale (NIHSS) was assessed at each visit. Disability was measured using the modified Rankin Scale (mRS) by experienced neurologists. Patients with disabilities due to other causes were not included in the study. Global cognitive function was evaluated by a neuropsychologist with over 10-year experience using the Mattis Dementia Rating Scale (MDRS) and Mini-Mental State Examination (MMSE). The cognitive evaluation included the Trail Making Test Part A (TMT A) and Part B (TMT B) tests as a measure of executive function [14]. The cut-off values for normality of each cognitive test were determined according to the patients’ education level and age respectively. These values were for the MDRS scores, if <12 years of education, ≥122, ≥120, ≥118, and ≥114 at age <78, 78–80, 81–83, and 84–86 years; if ≥12 years, ≥133, ≥132, ≥131, ≥130, ≥129, and ≥128, at age <72, 72–74, 75–77, 78–80, 81–83, and 84–86 years; for the MMSE scores, if <9 years of education, ≥25; if 9–13 years, ≥27 and if >13 years, ≥28; for the TMT A time, if <6 years of education, ≤54, ≤60, and ≤90s at age <70, 70–79, and ≥80 years; if 6–11 years, ≤48 and ≤76s at age <80 and ≥80 years; if ≥12 years, ≤44 and ≤64s at age <80 and ≥80 years; for the TMT B time, if <6 years of education, ≤186, ≤196, and ≤212s; if 6–11 years, ≤133, ≤187, and ≤212s and; if ≥12 years, ≤129, ≤136, and ≤189s, always at age <70, 70–79, and ≥80 years.
Magnetic resonance imaging data
Details of the magnetic resonance imaging (MRI) protocol and sequences used in this study have been previously detailed extensively [15,16]. In brief, the MRI examination included three-dimensional millimetric T1-weighted (3D-T1), fluid-attenuated inversion recovery (FLAIR), diffusion weighted images (DWI), and T2*-weighted gradient-echo images or susceptibility weighted (SW) images. In the present study, cerebral lesions were systematically assessed by an experienced neuroradiologist blinded to the clinical characteristics of the patients and according to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) criteria [17].
Lacunes were identified on 3D-T1 images by two expert neurologists (H.C., L.G.) or a neuroradiologist (R.Z.) as round or ovoid, subcortical, fluid-filled cavities (with a signal similar to that of CSF) of diameter from 3 to 15mm. A special attention was given to differentiate these lesions from enlarged perivascular spaces according to their size, shape, and rim. Recent small subcortical infarcts were sought on DWI images obtained at last visit as an hyperintense lesion located in the territory of a perforating artery and less than 20 mm in its maximum diameter. The number of microbleeds was determined after reading T2* or SW images after counting focal, small, rounded or circular, hypointense lesions within the brain parenchyma with clear margins and ranging from 2 to 10mm in diameter.
The volume of WMHs was finally calculated in each subject using the Brain Intensity AbNormality Classification Algorithm (BIANCA, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) [18] segmentation tool, a fully automated, non-parametric, learning supervised method based on the k-nearest neighbor algorithm which was previously adapted to CADASIL patients [16]. The total volume of WMHs was normalized to the intracranial cavity (ICC) in each patient [normalized WMHs volume = (WMHs volume / ICC volume) × 100%].
The Fazekas scale was used separately for rating WMHs visually in different locations. Periventricular WMHs were graded as absent (grade 0), caps (grade 1), smooth halos (grade 2), or irregular and extending into the subcortical white matter (grade 3). Deep WMHs were graded as absent (grade 0), punctate foci (grade 1), early-confluent (grade 2), or confluent (grade 3) [19]. WMHs in the temporal poles were assessed separately also using this latter grading.
Finally, brain atrophy was assessed after the segmentation of the whole brain tissue and of the ICC as previously reported [20]. This allowed to calculate the brain parenchymal fraction as the ratio of the brain tissue volume to the ICC volume.
Statistical analysis
All patients over 65 years of age at their last follow-up visit were dichotomized in two groups according to their clinical status. They were considered as Nint group if they fulfilled all the following criteria: (1) no focal deficit at neurological examination and NIHSS at 0; (2) lack of any significant disability and mRS ≤1; (3) no cognitive impairment and normal MDRS score according to age and education level or normal scores for both MMSE, TMT A time and TMT B time when the MDRS score was not available [21]. Patients with transient and benign manifestations such as migraine with aura or mood changes were included in the Nint group. All other patients older than 65 years belonging to the cohort were considered to present some permanent neurological symptoms (Nps) or signs of the disease and grouped separately.
Fisher’s exact test was used to compare the dichotomous variables between groups, while Mann-Whitney U test (for data with non-normal distributions) and Student’s t-test (for data with normal distributions) was used for the continuous variables. All analyses were performed blinded to the participant identifying information. Statistical significance was set at a probability value of <0.05. All statistical analysis was performed with an SPSS package version 22.0 for Windows (IBM Co., Armonk, NY, USA).
Results
One hundred and twenty-nine patients whose age was higher than 65 years at their last follow-up visit were selected from the entire cohort. Among them, 60.5% were women. The mean age at last visit was 72±5 years (median age, 72; interquartile range, 68 to 74). Eighty-nine patients (69.0%) were identified as the first family case at inclusion.
Among the 129 elderly selected CADASIL patients, 23 (17.8%) individuals were considered as Nint. Their main characteristics are summarized in Table 1. Thirteen of them were diagnosed after MRI evaluation of attacks of migraine with aura, one after the occurrence of repeated isolated auras, three had stroke and one had TIA, three had an MRI after a depressive episode associated in one case with chronic headache, and one patient had syncope-like episodes. Only in one patient, MRI and genetic testing were performed after the diagnosis of CADASIL in her brother who had a recent ischemic stroke. Vascular risk factors were present in 65% of Nint subjects, hypertension was diagnosed in 26% of them.
The comparison between Nint and Nps patients is detailed in Table 2. The results showed that age, the sex ratio, and frequency of vascular risk factors did not differ between the two groups. Migraine with aura were significantly more frequent in the Nint than in the Nps group. Notably, Nint patients had significantly less stroke than the Nps patients. Transient episode of mood disturbance, such as episodes of anxiety or depression that needed a medical treatment, in the Nint group were significantly less frequent than in the Nps group. Also, the different clinical scores (mRS, Barthel index, NIHSS, MMSE, and MDRS) obtained in each group confirmed the large clinical integrity of Nint patients compared to the Nps group. The same differences were observed from inclusion in the study when only baseline data were considered in the analysis (Supplementary Table 1).
The analysis of MRI parameters detailed in Table 3 showed that the volume of WMHs did not differ between the Nint and Nps group, nor did the Fazekas scores obtained separately for the periventricular and deep WMHs. Conversely, the load of lacunes and microbleeds largely differ between the two groups. Nint patient also present with a higher normalized brain volume than Nps patients. These results did not differ when only patients from distinct families were considered in the analysis (comparison restricted to only the first family members participating to the cohort study and considered as “index” patients, see Supplementary Tables 2 and 3).
The main imaging findings in the 23 Nint patients are summarized using FLAIR images on Figures 1 and 2. These data clearly confirmed the large variability in the load of WMHs in Nint patients whose normalized WMHs volume was ranging from 1.0% to 13.3%. Eleven patients harbored a variant located in EGFr domains 1-6, while 12 patients harbored a variant located in EGFr domains 7-34. The figures showed that patients with variants in EGFr domain 1-6 had a higher load of WMHs than patients with variants in EGFr domain 7-34 (normalized WMHs volume, 8.9% vs. 5.2%, P=0.027), especially in the temporal poles (WMHs score, 3 vs. 1, P=0.023). In the entire group (including both Nint and Nps individuals), patients with variants in EGFr domain 1-6 also had a higher load of WMHs in the temporal poles than the others with variants in EGFr domain 7-34 (WMHs score, 3 vs. 2, P<0.001), but the total lesion load did not differ between the two groups (normalized WMHs volume, 8.0% vs. 6.3%, P=0.234). The results did not change when only the subgroup of Nint patients who never had a stroke (n=12) was compared to all other patients older than 65 years (n=117, see Supplementary Tables 4 and 5).
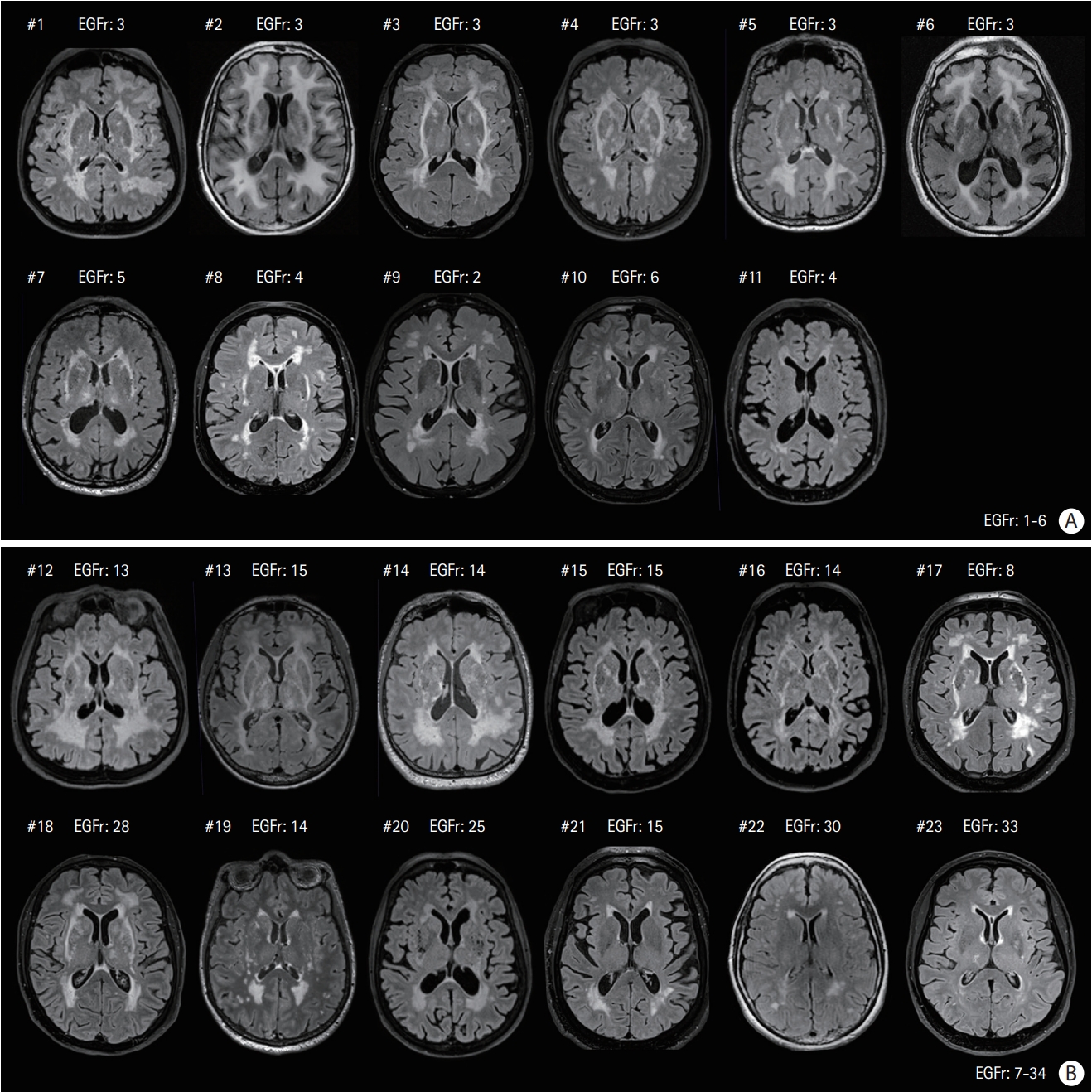
Magnetic resonance fluid-attenuated inversion recovery images of the 23 elderly neurologically intact (Nint) patients at the level of basal ganglia. (A) Eleven patients had their cysteine mutation in epidermal growth factor-like repeat (EGFr) domain 1-6 (Patient #1 to #11), (B) 12 others in EGFr domain 7-34 (Patient #12 to #23). Only three patients (Patient #1, #2, and #5) were from the same family. The patients were sorted according to their white matter hyperintensities load (from the highest to the lowest) in the two EGFr groups, respectively.
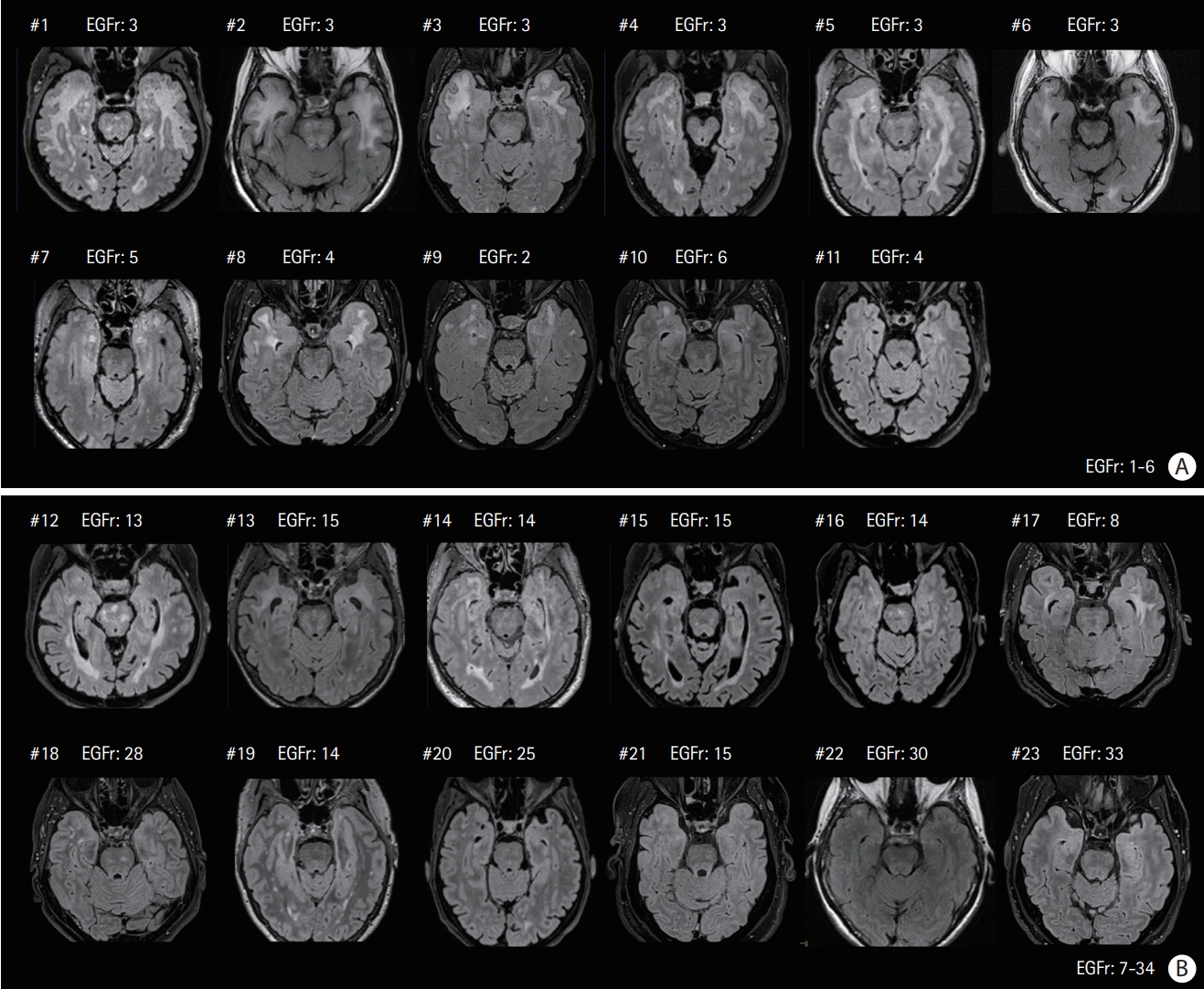
Magnetic resonance fluid-attenuated inversion recovery images of the neurologically intact (Nint) patients already presented in Figure 1 (same order) at the level of temporal pole. (A) Eleven patients had their cysteine mutation in epidermal growth factor-like repeat (EGFr) domain 1-6 (Patient #1 to #11), (B) 12 others in EGFr domain 7-34 (Patient #12 to #23).
Discussion
In this large prospective cohort of patients diagnosed with CADASIL, we observed that 17.8% of individuals can remain Nint despite their age extending beyond 65 and up to 76 years. Only anecdotal elderly cases up to 86 years have been previously described in the literature as “minimally symptomatic,” “pauci-symptomatic,” or “with no history of stroke or vascular cognitive decline.” [9,22-30] In the present study, the drastic selection of Nint cases from a large sample of consecutive elderly patients allows, for the first time, to delineate the spectrum of imaging and genetic characteristics associated with neurological preservation despite aging in CADASIL.
In the Nint group, 14 individuals already suffered attacks of migraine with aura or isolated auras. This frequency is twice that observed in the Nps group and much higher than the average frequency of 40% usually reported in cohorts of symptomatic CADASIL patients [31,32]. This is possibly related to our selection of Nint patients among cases recruited in a clinical center, who were mostly symptomatic and thus diagnosed after mild or transient clinical manifestations. Also, this high prevalence of migraine with aura in Nint elderly individuals further supports the absence of link between migraine with aura and clinical disability or cognitive decline in CADASIL [31,33]. Other transient clinical manifestations were depression episodes in two Nint cases, possibly favoured by the presence of confluent white matter abnormalities [34]. Interesting, only a single Nint woman was totally asymptomatic and diagnosed after her brother had a stroke at young age, which led to genetic testing. The results did not change when only “index” patients were considered in the analysis (supplementary data). Finally, we did not observe a significant difference in the frequency of cardiovascular risk factors between the Nps and Nint groups, suggesting that these factors are not involved in this clinical contrast. Conversely, Nint patients were more educated than Nps patients, thus whether additional protective factors related to differences in the level of regular exercise, diet, behavior, access to care, health awareness or to the environment cannot be ruled out [35].
As expected, Nint elderly patients had much less lacunes and microbleeds as well as less cerebral atrophy than Nps patients. These imaging markers are strongly related to the development of disability and dementia in CADASIL patients [13,36]. In contrast, the global amount of WMHs did not differ between the Nint and Nps groups. This discrepancy suggests that although the disease has visible effects in the white matter, the development of lacunes and loss of cerebral tissue along aging does not occur or remains limited in most Nint patients. In line, accumulating data support that some WMHs in CADASIL may result more from water accumulation than from myelin or axonal loss as observed in ischemic cerebral tissue [37,38].
The location of NOTCH3 mutation in different EGFr domains might be involved in this relative preservation despite aging. About half of Nint CADASIL patients harbored cysteine mutations within EGFr domain 7-34. This is higher than the average frequency of such mutations in European cohorts of symptomatic diagnosed cases [3]. Since a selection bias related to the inclusion of only survivors aged more than 65 years cannot be excluded in our cohort, this proportion might be even underestimated. Recent pathology study of skin biopsies suggested that patients with an EGFr 7-34 variant might have less NOTCH3 extracellular domains accumulation in the wall of their brain vessels than patients with an EGFr 1-6 variant [39]. This would explain the higher prevalence of these mutations in the general population with a much larger number of silent or mild forms of the disease [3,40]. In the present study, the location of NOTCH3 variants within EGFr domains did not differ between Nint and Nps patients. Therefore, if the impact of mutation location on the level of NOTCH3 accumulation is actually proven at cerebral vascular level, other additional factors are obviously involved. Various protection mechanisms may contribute to this relative preservation at multiple level, such as, genetic or hormonal modulation of brain tissue susceptibility to chronic hypoperfusion, anatomical or physiological characteristics of the cerebral microvascular network or processes involved in the triggering and adaptation of cerebral blood flow autoregulation at tissue level.
Obviously, among elderly Nint patients, a large amount of WMHs involving both temporal lobes was observed in most cases having a mutation in EGFr domain 1-6 while the extent of WMHs appeared much less and the temporal poles largely spared in presence of a mutation in EGFr domain 7-34. At the opposite extreme, very beginning and limited WMHs were detected in a 66-year-old patient having a cysteine mutation in EGFr domain 30 (case 22) but such beginning WMHs were also detected in a 73-year-old patient who had a mutation in EGFr domain 4 (case 11). Altogether, these results suggest that the location of NOTCH3 mutation might modulate the mechanisms involved in the development of WMHs, especially in the white matter adjacent to the cortex as within the temporal poles. The factors needed to delay by several decades the appearance of WMHs in some individuals remain also unknown.
The strengths of this study are multiple. The selected sample of elderly CADASIL patients is large. Each case was extensively investigated. Both clinical results, MRI data and the location of their mutation in the NOTCH3 gene were analyzed in detail. The collection of data was obtained by experienced clinicians over 15 years and always using the same imaging and clinical parameters. The study has also some limitations. It is based on data driven from the hospital, consequently, in the Nint group, totally asymptomatic individuals are probably largely underestimated. On the opposite side, in the Nps group, elderly cases who were severely disabled or dependent are also most likely underrepresented. They have more difficulties to participate in research activities and to travel to the referral center. The most severe CADASIL cases who died before the age of 65 years are also not accounted in such a comparison study. Besides, due to the very long duration of the disease, we didn’t analyze all treatments that may have been used over decades in all individuals. In addition, subtle psychiatric disturbances might have not been taken into account when defining “intact” neurological status. Finally, the interpretation of our data based on imaging data, particularly in Nint patients should remain cautious in the absence of pathological examination.
Conclusions
We found that nearly one in five CADASIL patients can remain Nint after the age of 65 years. Their different clinical and imaging profile cannot be fully explained by the location of NOTCH3 mutation inside or outside EGFr domains 1-6. In some Nint individuals, severe ischemic lesions do not develop conversely to WMHs that can become extensive. In others, all types of cerebral lesions, including WMHs appear extremely limited and might be delayed by several decades for unknown reasons. The factors involved in this relative cerebral tissue preservation along aging remain to be determined.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.01578.
Comparison of baseline characteristics between Nint and Nps patients
Comparison of demographic and clinical data between “index” Nint and Nps patients
Comparison of imaging data between “index” Nint and Nps patients
Comparison of demographic and clinical data between Nint patients who never had a stroke and the other patients
Comparison of imaging data between Nint patients who never had a stroke and the other patients
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the French Ministry of Health (Regional and National PHRC AOR 02-001) and Research (Agence National de la Recherche, ANR, RHU TRT_ cSVD), Association de Recherche en NEurologie Vasculaire. Ruiting Zhang was funded by the China Postdoctoral Council (Grant no. PC2020117).
We warmly thank the team in charge of formatting and cleaning the database using multiple information collected over 14 years from different sources and which made possible this study, particularly Professor Sylvie Chevret and Mrs Claire Pacheco (INSERM UMR1136). We thank very much to Dr Dominique Hervé and Nassira Alili who collected important clinical data for this study, Mr Abbas Taleb for collecting most of information along the cohort study, Mrs Sonia Reyes who is responsible for neuropsychological assessments, Mrs Aude Jabouley, Carla Machado who performed a large number of cognitive evaluation in the cohort, Mrs Solange Hello who managed and organized the appointment of multiple family members involved in the study, Mrs Nathalie Gastelier and Fanny Fernandes, the research managers in charge of the Cohort Study. We thank the CADASIL France Association for their help and permanent support.