Serum 25-Hydroxyvitamin D Deficiency in Ischemic Stroke and Subtypes in Indian Patients
Article information
Abstract
Background and Purpose
Vitamin D deficiency is common across all age groups and may contribute to cardiovascular diseases. Serum 25-hydroxyvitamin D deficiency causing ischemic stroke has been documented in recent reports.
Aim
To investigate the association of serum 25-hydroxyvitamin D deficiency with ischemic stroke and subtypes.
Methods
We recruited 250 consecutive ischemic stroke patients and 250 age and sex matched controls attending the Department of Neurology, at Yashoda hospital, Hyderabad, India, from January 2011 to December 2012. All ischemic stroke patients underwent stroke subtyping. We measured 25-hydroxyvitamin D by chemiluminescence test, serum calcium, phosphorus, alkaline phosphatase, and C-reactive protein (CRP) in cases and controls.
Results
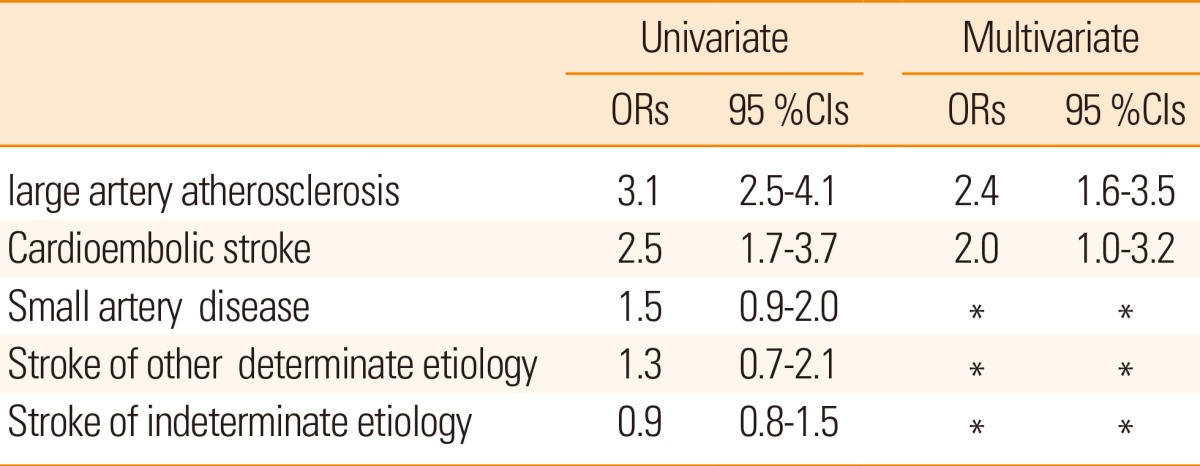
Out of 250 stroke patients, 190 (76%) were men and mean age was 58.4±11.1 years (age range-26-89 years). 25-hydroxyvitamin D deficiency was observed in 122 (48.8%) stroke patients and 79 (31.6%) controls (P=0.001). Among stroke patients, serum 25-hydroxyvitamin D deficiency was found in 54.9% (50/91) of patients with large artery atherosclerosis, 54% (20/37) in cardioembolic stroke, 44.4% (20/45) in small artery diseases, 42.8% (15/35) in stroke of other determined etiology and 40.4% (17/42) in stroke of un-determined etiology. Multiple logistic regression analysis showed an independent association of 25-hydroxyvitamin D deficiency with ischemic stroke (odds ratio: 1.6; 95% CI 1.2-2.8). The association was strongest with large artery atherosclerosis (odds ratio: 2.4; 95% CI 1.6-3.5) and cardioembolic stroke (odds ratio: 2.0; 95% CI 1.0-3.2).
Conclusions
We found that 25-hydroxyvitamin D deficiency had an independent association with ischemic stroke. The association was established in large artery arthrosclerosis and cardioembolic stroke.
Introduction
Stroke is a sudden onset of focal neurological deficit, a major cause of morbidity and mortality and the second leading cause of death worldwide.1 Stroke has a heterogeneous etiology, caused by modifiable and un-modifiable risk factors. Recent studies have strongly suggested an association of deficiency of 25-hydroxyvitamin D with ischemic stroke2,3 and cardiovascular disease.4,5
Vitamin D is a 9,10-seco steroid and the most common forms in humans are vitamin D3 (cholecalciferol) and Vitamin D2 (ergocalciferol).2 Vitamin D is essential for the human body to maintain a balance between calcium and phosphorus. Inadequate vitamin D can cause weakness, reduced bone mineralization, increased bone loss and hip fracture6 and its prevalence is high in both hemispheric populations.7 Serum 25-hydroxyvitamin D is the circulating form of vitamin D with a half life of 2 to 3 weeks and is converted to the active form -1,25-dihydroxy vitamin D3 in the kidneys.8 25-hydroxyvitamin D is a marker of vitamin D status in the human body.9,10 Some population based studies have shown that 40%-45% of Indians have 25-hydroxyvitamin D deficiency in India.11 We aim to investigate the association between serum 25-hydroxyvitamin D deficiency and ischemic stroke and its subtypes in Indian patients. There is no study, so far, on the association of serum 25-hydroxyvitamin D deficiency with increased risk of stroke in Indian patients.
Methods
Two hundred fifty patients with ischemic stroke enrolled consecutively in Yashoda hospital, which is a major referral center in the state of Andhra Pradesh. Two hundred fifty age and sex matched controls were recruited from healthy volunteers with no prior history of stroke or transient ischemic attacks. This cohort consisted of patient attendees and people volunteering for blood donation. The study period was between January 2011 and December 2012. This study is a part of ReVDAS (Role of 25-hydroxvitamin D in Acute Ischemic Stroke) study. This study was approved by the Institutional Ethics Committee and informed consent was obtained from controls and patients and if patients were severely ill, consent was taken from their relatives.
Selection of cases
Stroke patients met the following criteria: first ischemic stroke, admitted within 7 days of stroke onset. Stroke was defined according to the World Health organization as "rapidly developing clinical signs of focal/global disturbance of cerebral function, with no apparent cause other than of vascular origin."12 Cerebral infarction was diagnosed on the basis of the first Computer tomography (CT) or Magnetic Resonance Imaging (MRI) brain scan. If patients had a normal CT scan brain, then ischemic stroke was diagnosed based on diffusion weighted MRI.
Stroke subtype analysis
All stroke patients underwent CT scan of brain (to rule out hemorrhagic stroke) initially, followed by MRI of brain. Magnetic Resonance Angiography (MRA), Transthoracic echocardiography (TTE) or Transesophageal echocardiography (TEE), non-invasive vascular imaging (Carotid Doppler) were done in all patients. Ischemic stroke subtypes were classified as large artery atherosclerosis, cardioembolic stroke, small vessel disease (lacunar stroke), stroke of other determined etiology and stroke of undetermined etiology.13 If the etiology was not clear, then additional tests-serum fibrinogen, antithrombin III, protein C, protein S, antinuclear and anticardiolipin antibodies were also done. Lipid profile and serum homocysteine estimation were done in all patients.
Standardized questions were modified from the behavioral risk factor surveillance system,14 by the Centers for Disease Control and Prevention. Height, weight, and blood pressure were measured, fasting blood samples were collected to estimate blood glucose, serum lipid profiles, calcium, phosphorus, alkaline phosphatase and C-reactive protein (CRP) for cases and controls.
Definition for hypertension by JNC VII (Joint National Committee)-defined as a systolic blood pressure >140 mmHg and/or a diastolic blood pressure >90 mmHg based on the average of 2 blood pressure measurements at the time of admission, or a patient's self-reported history of hypertension or antihypertensive use, supported by documents.15 Diabetes was diagnosed if fasting plasma glucose was >110 mg/dL or patient was on anti-diabetic medications.16 As per guidelines of National Institute of Health (NIH), patients with serum cholesterol levels >200 mg/100 mL or those on anticholesterol medication were considered as having hypercholesterolemia.17 Smokers were defined as those reporting daily smoking. Ex-smokers and occasional smokers were classified as nonsmokers.18 Alcoholics were defined as those in whom the alcohol consumption was >50 g/day (equivalent to 500 mL [2 drinks] of wine, 1,000 mL of beer, or N5 drinks [units] of spirits).19 Body Mass Index (BMI) values from 25.0-30.0 were taken as overweight and BMI values >30 were taken as obese.20
Blood collection
Blood collection was done at the time of enrollment of cases and controls; 5 mL blood sample was used for estimation of 25-hydroxyvitamin D. We used chemiluminescent microparticle immunoassay (CMIA) with automated instruments for estimation of 25-hydroxyvitamin D. Values≤20 ng/mL were diagnosed as 25-hydroxyvitamin D deficiency.10,21,22 Values from 11-20 ng/mL were considered mild and <10 ng/mL were diagnosed as severe vitamin D deficiency.
Statistical analysis
Statistical analysis was performed using SPSS 14.0 software (Chicago, IL, USA). Mean +SD (Stranded Deviation) were calculated. The paired 't' test was applied to test the differences in continuous variables and McNemar test was applied to study the association in proportions. We estimated odds ratio (OR) and the resulting 95% CI for the matched case-control pairs. Multiple logistic regression was performed before and after adjustment for potential confounders -age, gender, hypertension, diabetes, smoking, alcoholism, hyperlipidemia and obesity. All tests were two sided and P value <0.05 were considered statistically significant.
Results
Out of 250 subjects, men were 190 (76%), mean age was 58.4±11.1 years (age range - 24-89 years). Hypertension was significantly more common in stroke patients (144 [57.6%]) compared to controls (40 [26.6%]; P<0. 0001) as was diabetes (74 [49.3%] of stroke patients vs. 31 [24%] controls; P<0.0001). Positive CRP was noted in 156 (62.4%) stroke patients while seen only in 83 (33.3%) controls (P<0.0001). 25-hydroxyvitamin D deficiency was more prevalent in stroke patients 22 [48.8%]) than controls 79 (31.6%) which was statistically significant (P=0. 0001). Significantly decreased mean serum calcium (8.8±2.6) (mg/dL) and phosphorus (3.6±1.6) (mg/dL) levels were found in stroke patients compared to controls. Mean alkaline phosphatase level was significantly increased in stroke patients (112.1±38.6) (µ/L) compared to controls (85.5±21.5)(µ/L) (P<0.0001) (Table 1).
We subdivided the 25-hydroxyvitamin D deficiency group into mild (10.1-20.0 ng/mL) and severe deficiency (below 10 ng/mL) in stroke patients and controls. We found significantly higher proportions in stroke patients, of both mild (65 [26%] vs. 45 controls [19.6%], P=0.002) and severe 25-hydroxyvitamin D deficiency (57 [22.8%] vs. 34 controls [12%], P=0.02).
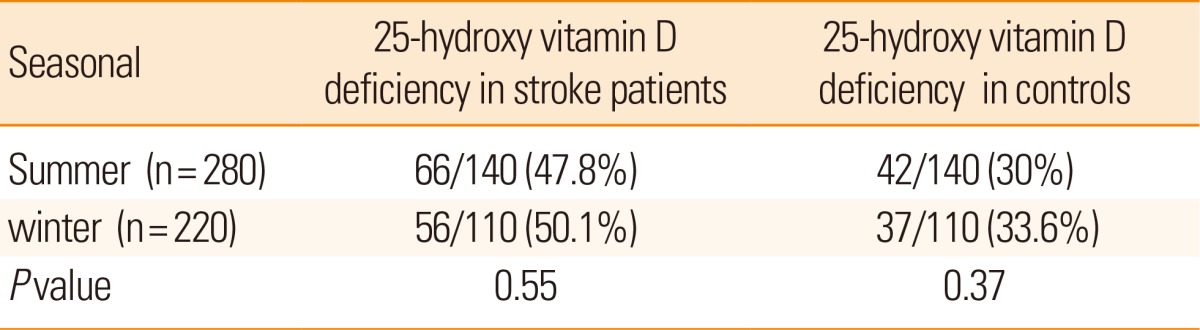
We compared the values of samples taken during summer (samples were collected from March to September) and winter (samples were collected from October to February). There were no significant differences in prevalence of 25-hydroxy vitamin D deficiency between summer and winter samples in cases (P=0.5) and controls (P=0.3) (Table 2). But in both the seasons the prevalence of 25-hydroxyvitamin D deficiency was significantly higher among stroke patients compared to controls (<0.02).
Among stroke subtypes, 25-hydroxyvitamin D deficiency was present in 50 patients (54.9%) with large artery atherosclerosis, 20 patients (54%) with cardioembolic stroke, 20 patients (44.4%) with small artery disease, 15 patients (42.8%) with stoke of other determined etiology and 17 patients (40.4%) with stroke of un-determined etiology.
In the 20 patients with cardioembolic stroke and 25 hydroxyvitamin D deficiency, the underlying cardiac disease was varied and included history of myocardial infarction (4 patients), atrial fibrillation (3 patients) congestive heart failure (4 patients), akinetic left ventricular segment (2 patients ) ascending aorta stenosis (2 patients), mitral valve stenosis (2 patients), and rheumatic heart disease (3 patients).
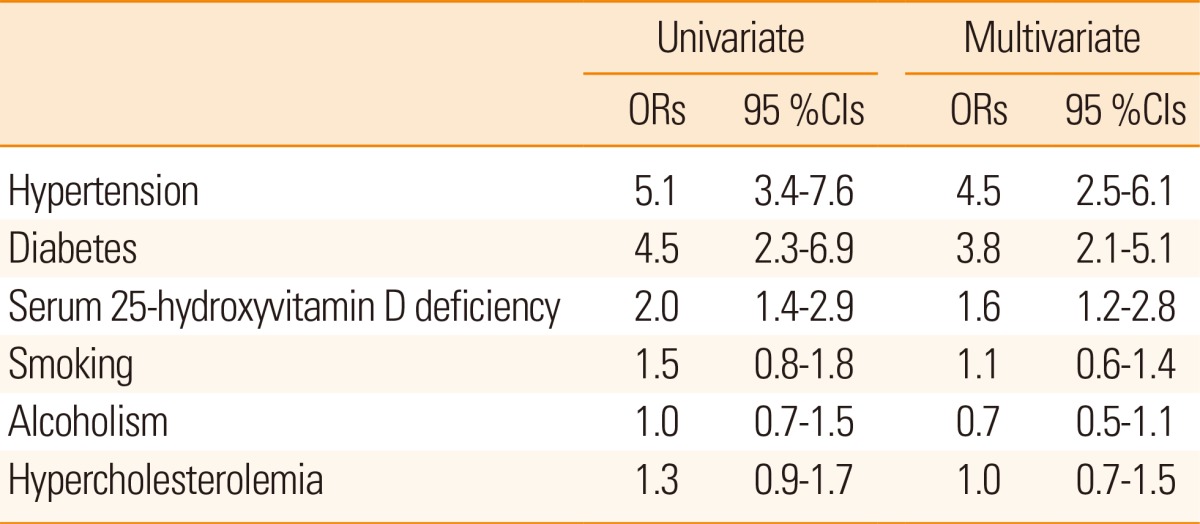
On comparing the risk factors with stroke, univariate analysis demonstrated maximum risk with hypertension and diabetes followed by 25-hydroxyvitamin D deficiency. 25-hydroxyvitamin D deficiency showed an independent association with ischemic stroke (Table 3).
On evaluation of stroke subtypes 25-hydroxyvitamin D deficiency was independently association with large artery atherosclerosis and cardioembolic stroke (Table 4).
Discussion
In our study, we found a significant association between 25-hydroxyvitamin D deficiency and ischemic stroke and established an independent association. Similar results have been found from the western part of the world.2,3,23-26 We noted deficiency of 25-hyroxyvitamin D in 54.9% of stroke patients with large artery atherosclerosis. A similar association of hypovitaminosis D with large artery atherosclerosis and small artery disease has been described earlier.25 A recent study showed low 25-hydroxyvitamin D was significantly associated with increasing intimal media thickness and carotid plaques in individuals.27 We also found a significant association of 25-hyroxyvitamin D deficiency with cardioembolic stroke. Several studies have shown a strong association of vitamin D deficiency with cardiovascular disease.28-30 Giovannucci et al.31 demonstrated low levels of 25-hydroxyvitamin D as a high risk factor for myocardial infarction. Lower 25-hydroxyvitamin D concentration was shown to be an independent risk factors for atherosclerosis, coronary calcification32 and cardiovascular death.33 However some studies have found no association between vitamin D and cardiovascular disease.34,35
The mechanism of deficiency of vitamin D and atherosclerosis is not fully understood. Li et al.36 observed that vitamin D regulated blood pressure by suppressing the renin angiotensin system. Aihara et al.37 demonstrated vascular effects of vitamin D with inhibition of thormobosis38 and reduction in arterial calcification.39 In addition smooth muscle cells and lymphocytes express receptors for vitamin D and convert circulating 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D. 1,25-hydroxy vitamin D in turn reduce the proliferation of lymphocytes and the production of cytokines.39,40 This anti-inflammatory effect may have a protective role as there is increasing evidence that systemic inflammation leads to atherosclerosis.41
In our study we also observed significantly higher proportion of stroke patients with elevated levels of alkaline phosphatase and decreased phosphate levels compared with control subjects. This is advocated by Nibet et al.42 and Preece et al.43 Decreased serum phosphate and increased alkaline phosphatase are caused by Vitamin D deficiency.44,45 Alkaline phosphatase may contribute to atherosclerosis by promoting vascular calcification. Elevated alkaline phosphatase has also been shown to be an independent predictor of mortality after ischemic stroke.46
CRP an inflammatory marker and an independent risk factor for acute ischemic stroke.47,48 In our study we found significant association deficiency of 25-hydroxyvitamin D with CRP positive in stroke patients compared to controls. Recent studies have advocated that CRP levels are elevated in deficiency of vitamin D.31 This furthers emphasizes the role of vitamin D in reducing inflammation and thus reducing atherogenesis.
The strength of this study is that both cases and controls were collected from a single center with same ethnic background and 25-hydroxyvitamin D analysis was done in one lab. We have also analyzed the association of 25-hydroxyvitamin D deficiency with stroke subtypes.
Age may impact vitamin D levels. Gillor et al found that with increasing age 25-hydroxyvitamin D decreases,49 as capability of skin to produce pre-vitamin D after ultraviolet (UV) B irradiation declines with age.50 In our study this effect is nullified as both cases and controls were age and sex matched.
We did not perform seasonal adjustment for vitamin D levels, as in our study. we did not find any significant difference between levels during winter and summer. Similar results were found in healthy subject in previous studies from India.51,52 This difference from west may be due to the fact that as a tropical country the temperature fluctuations are ±10 degrees centigrade and may not impact vitamin D levels.
Conclusions
This study established that deficiency of 25-hydroxyvitamin D had an independent association with ischemic stroke-especially large artery atherosclerosis and cardioembolic stroke. A single serum measurement of this compound could be a useful marker in epidemiologic studies. The role of vitamin D deficiency as a direct causative factor of stroke has to be established for advocating vitamin D usage for stroke prevention. Large scale interventional studies are required to confirm these findings.
Acknowledgements
Our sincere thanks to the Dr.G.S. Rao, Managing Director, Yashoda group of hospitals and Dr.A.Lingaih, Director of Medical services for their generous support to carry out this study in Yashoda Hospital. Hyderabad.
Notes
The authors have no financial conflicts of interest.