Association of Lipids, Lipoproteins, and Apolipoproteins with Stroke Subtypes in an International Case Control Study (INTERSTROKE)
Article information
Abstract
Background and Purpose
The association of dyslipidemia with stroke has been inconsistent, which may be due to differing associations within etiological stroke subtypes. We sought to determine the association of lipoproteins and apolipoproteins within stroke subtypes.
Methods
Standardized incident case-control STROKE study in 32 countries. Cases were patients with acute hospitalized first stroke, and matched by age, sex and site to controls. Concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (apoA1), and apoB were measured. Non-HDL-C was calculated. We estimated multivariable odds ratio (OR) and population attributable risk percentage (PAR%). Outcome measures were all stroke, ischemic stroke (and subtypes), and intracerebral hemorrhage (ICH).
Results
Our analysis included 11,898 matched case-control pairs; 77.3% with ischemic stroke and 22.7% with ICH. Increasing apoB (OR, 1.10; 95% confidence interval [CI], 1.06 to 1.14 per standard deviation [SD]) and LDL-C (OR, 1.06; 95% CI, 1.02 to 1.10 per SD) were associated with an increase in risk of ischemic stroke, but a reduced risk of ICH. Increased apoB was significantly associated with large vessel stroke (PAR 13.4%; 95% CI, 5.6 to 28.4) and stroke of undetermined cause. Higher HDL-C (OR, 0.75; 95% CI, 0.72 to 0.78 per SD) and apoA1 (OR, 0.63; 95% CI, 0.61 to 0.66 per SD) were associated with ischemic stroke (and subtypes). While increasing HDL-C was associated with an increased risk of ICH (OR, 1.20; 95% CI, 1.14 to 1.27 per SD), apoA1 was associated with a reduced risk (OR, 0.80; 95% CI, 0.75 to 0.85 per SD). ApoB/A1 (OR, 1.38; 95% CI, 1.32 to 1.44 per SD) had a stronger magnitude of association than the ratio of LDL-C/HDL-C (OR, 1.26; 95% CI, 1.21 to 1.31 per SD) with ischemic stroke (P<0.0001).
Conclusions
The pattern and magnitude of association of lipoproteins and apolipoproteins with stroke varies by etiological stroke subtype. While the directions of association for LDL, HDL, and apoB were opposing for ischemic stroke and ICH, apoA1 was associated with a reduction in both ischemic stroke and ICH. The ratio of apoB/A1 was the best lipid predictor of ischemic stroke risk.
Introduction
The association of lipids, lipoproteins, and apolipoproteins with stroke is more complex than that reported for acute myocardial infarction (AMI), with inconsistent findings among epidemiological studies [1]. In studies that do report an association of lipoproteins and apolipoproteins with stroke, the magnitude of the association is lower than reported for AMI [2-6] and appears to differ by stroke etiology [7-23]. Meta-analyses of observational studies report opposing directions of association of total cholesterol, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) with ischemic stroke compared to intracerebral hemorrhage (ICH); for example, increasing LDL-C is associated with a higher risk of ischemic stroke but an inverse association with ICH.24 These opposing associations may explain null associations in stroke studies that did not complete routine neuroimaging, required for valid distinction between ischemic and hemorrhagic stroke [7]. Within ischemic stroke subtypes, there is also some evidence to suggest there may be differing associations of lipoproteins within etiological ischemic subtypes with some studies suggesting a stronger magnitude of association for large vessel atherosclerotic stroke, compared to small vessel or cardioembolism [25-28]. These differences may have clinically relevant implications for tailoring prevention strategies by ischemic stroke subtype, and, may contribute to global variations in frequency of stroke subtypes.
The INTERSTROKE study is the largest international epidemiologic study to evaluate the association of cholesterol, lipoproteins lipids and apolipoproteins with stroke categorized by routine neuroimaging [29]. We have previously reported that the apolipoprotein B (apoB)/apoA1 ratio was associated with 34% of the population attributable risk (PAR) for ischemic stroke [29]. In the current report, we describe the comparative association of cholesterol, lipoproteins, and apolipoproteins within stroke subtypes.
Methods
Study design/population
INTERSTROKE is a large international case-control study [30] conducted from March 2007 to September 2015 among 142 centers in 32 countries in Asia, Africa, Europe, North America, Middle East, Australia, and South America. Participants (n=26,919) were recruited, comprising 13,447 cases of acute first stroke and 13,472 controls (Appendix 1).
Cases were patients with acute first stroke admitted to hospital (within 72 hours) and within 5 days of symptom onset. Stroke was defined using the World Health Organization (WHO) clinical criteria for stroke [31]. Neuroimaging was completed in 99.9% of cases. Proxy respondents were used when patients were unable to communicate adequately. Controls were either community-based (54.7%) or hospital-based (45.3%). Specific approaches to identifying sources of community-based controls were not prespecified, as standardized approaches may not be feasible in all settings. However, each control was matched for sex and age (±5 years) with cases, extended to ±10 years for those >90 years. Eligibility criteria and sampling approach included in Supplementary Tables 1 and 2 [30]. Patients with ischemic stroke were classified etiologically according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [32,33] (large vessel, small vessel, cardioembolism, or undetermined) and reported by the local investigators 1 month after stroke, based on combined clinical assessment and results of available diagnostic tests [34,35]. We did not mandate other etiologic investigations (except electrocardiogram), as it may have resulted in a selection bias due to regional variations in availability of diagnostic testing. The modified Rankin Scale [36] was used to measure stroke severity. The study was approved by the ethics committees in all participating centers. All participants, or their proxy, provided informed written consent before taking part in the study.
Measurement of risk factors
Structured questionnaires were administered, and physical examinations were undertaken, in the same manner in cases and controls. Key vascular risk factors were measured in a manner consistent with the INTERHEART study [37]. These have been described in detail previously and included in the Appendix 2 for this paper. Blood samples were obtained and centrifuged within 24 hours of recruitment (78.0% of samples were collected within 48 hours of admission to hospital), and then separated immediately and frozen at –20℃ or –70℃, dependent on the availability of specific freezers. Samples were periodically shipped in nitrogen vapour tanks for storage at –80℃ in China, –80℃ in India, –80℃ in Turkey, or –160℃ in Canada. All reagents and kits used in each laboratory are reported in the Appendix 2. Concentrations of total cholesterol, HDL-C, LDL-C, apoA1, and apoB were measured with local analysers in each of four core laboratories. Concentrations of non-HDL-C were calculated as total cholesterol minus HDL-C.
Statistical analysis
Means and medians were calculated to summarise continuous variables and were compared by paired t-tests or appropriate paired non-parametric tests. Categorising of data by tertiles was based on control data. We used restricted cubic spline plots to explore the pattern of association of cholesterol, lipoproteins, and apolipoproteins with ischemic stroke, ischemic stroke etiological subtypes, and ICH. We fitted a restricted cubic spline function with four knots (5th, 35th, 65th, and 95th centiles), and completed a likelihood ratio test for non-linearity (included in figure legends). We used conditional logistic regression for all analyses of association of lipid fraction variables with stroke outcomes. All analyses were adjusted for age, hypertension, smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, cardiac risk factors (including atrial fibrillation), pre-admission statin use, and alcohol intake. To optimize power to detect associations for the analyses of the ICH subgroups, we supplemented the control group with controls for ischemic stroke to match additional controls to ICH cases (using the same matching criteria). Estimates of odds ratios (ORs) and accompanying 95% confidence intervals (CIs) are presented for every risk factor and their combinations. We estimated PAR% using the method described by Benichou and Gail [38]. CI calculations were based on this method using a logit transformation approach, apart from when PAR estimates were negative, in which case, conventional Wald type CIs were used. We tested P-interaction among lipid fractions (e.g., apoB vs. LDL-C) to evaluate differences in magnitude of association with ischemic stroke. Statistical analyses and graphics were produced with SAS version 9.2 (SAS, Cary, NC, USA) and TIBCO Spotfire S-Plus version 8.2 for Windows (Insightful, Seattle, WA, USA).
Data sharing statement
Data from INTERSTROKE are not available for public use.
Results
Participant characteristics
The mean±standard deviation (SD) age was 62.2±13.6 years. Non-fasting blood samples were obtained from 11,898 matched case-control pairs (n=23,796). Supplementary Table 3 reports baseline characteristics, proportion of stroke subtypes and diagnostic work-up, by region. Supplementary Table 4 reports the mean concentration of lipids, lipoproteins, apolipoproteins, and their ratios by sex and region in controls. Supplementary Table 5 report mean cholesterol, LDL-C, HDL-C, non-HDL-C, apoA1, apoB, non-HDL/HDL ratio, and apoB/A1 ratio in cases and controls. Patients with ischemic stroke had higher mean apoB, and lower apoA1 and HDL-C, than cases with ICH or controls. Mean total cholesterol was lower in cases, of both ischemic and ICH, than controls (Supplementary Table 5). Pre-admission statin use was reported in 11.2% of cases and 9.2% of controls.
Cholesterol, lipoproteins, and stroke
Total cholesterol had an inverse association with ischemic stroke (OR, 0.91; 95% CI, 0.87 to 0.95 per SD) and ICH (OR, 0.81; 95% CI, 0.77 to 0.86 per SD) (Figure 1, Supplementary Figures 1 and 2). Increasing LDL-C was associated with an increase in risk of ischemic stroke (OR, 1.06; 95% CI, 1.02 to 1.10 per SD), with an apparent threshold effect above approximately 3.5 mmol/L (Figure 2A) and associated with reduced risk of ICH (OR, 0.91; 95% CI, 0.86 to 0.96 per SD) (Figure 1). Increasing HDL-C was associated with a reduction in risk of ischemic stroke (OR, 0.75; 95% CI, 0.72 to 0.78 per SD) but increased risk of ICH (OR, 1.20; 95% CI, 1.14 to 1.27 per SD) (Figure 1). Within ischemic stroke subtypes, higher LDL-C levels were significantly associated with large vessel ischemic stroke (OR, 1.12; 95% CI, 1.02 to 1.23 per SD), ischemic stroke of undetermined cause (OR, 1.10; 95% CI, 1.02 to 1.19 per SD) but not significantly associated with other ischemic stroke subtype (Figures 1 and 3A). Increasing HDL-C was associated with a reduction in odds of all ischemic stroke subtypes (Figures 1 and 3B).
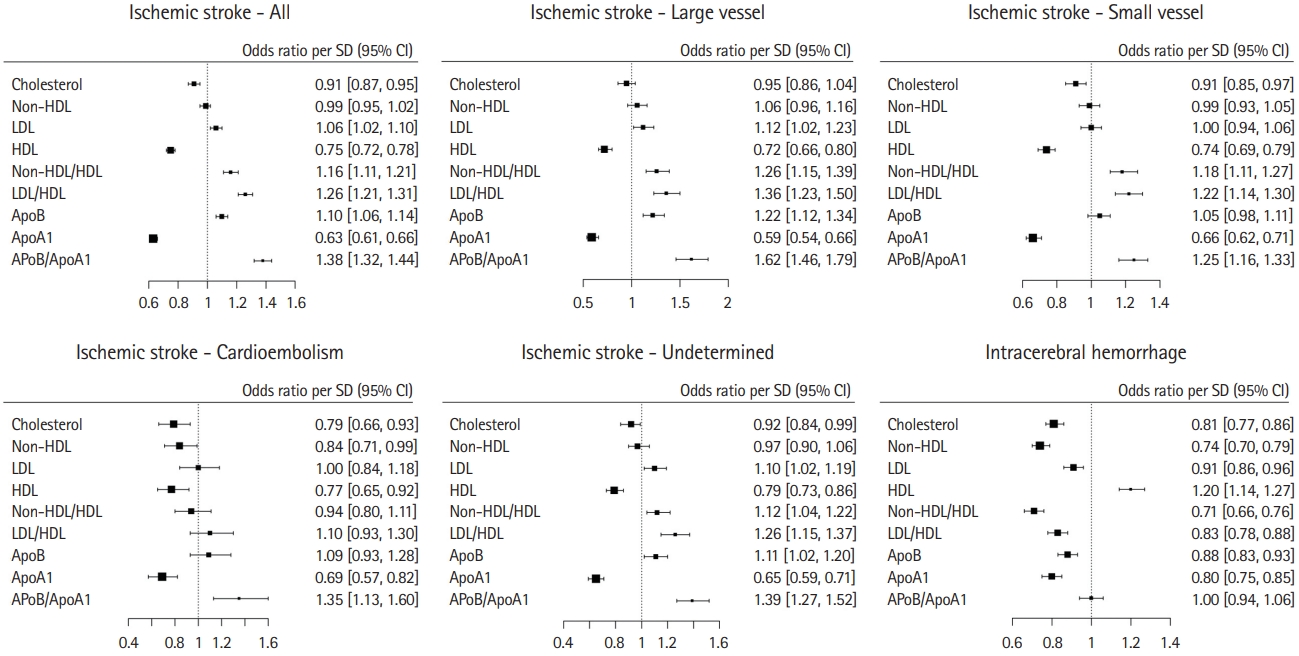
Association of total cholesterol, lipoproteins and apolipoprotein (Apo), and stroke. Forest plot for association of total cholesterol, non-high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), HDL-C, non-HDL/HDL ratio, LDL/HDL ratio, apoB, apoA1, and apoB/A1 ratio, and ischemic stroke, ischemic stroke subtypes, and intracerebral hemorrhage. Odd ratio and 95% confidence interval (CI) per standard deviation (SD) change.

(A) Association of lipoproteins with ischemic stroke and intracerebral hemorrhage (ICH). (B) Association of apolipoproteins with ischemic stroke and ICH. Restricted cubic spline plot of association of (A) lipoproteins (low-density lipoprotein cholesterol [LDL-C] and high-density lipoprotein cholesterol [HDL-C]) (X-axis) and (B) apolipoproteins (apoB and apoA1) (X-axis) with ischemic stroke and ICH. Spline curve truncated at highest and lowest 2% of values. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet risk score, psychosocial factors, waist-to-hip ratio, pre-admission statin, and alcohol intake).
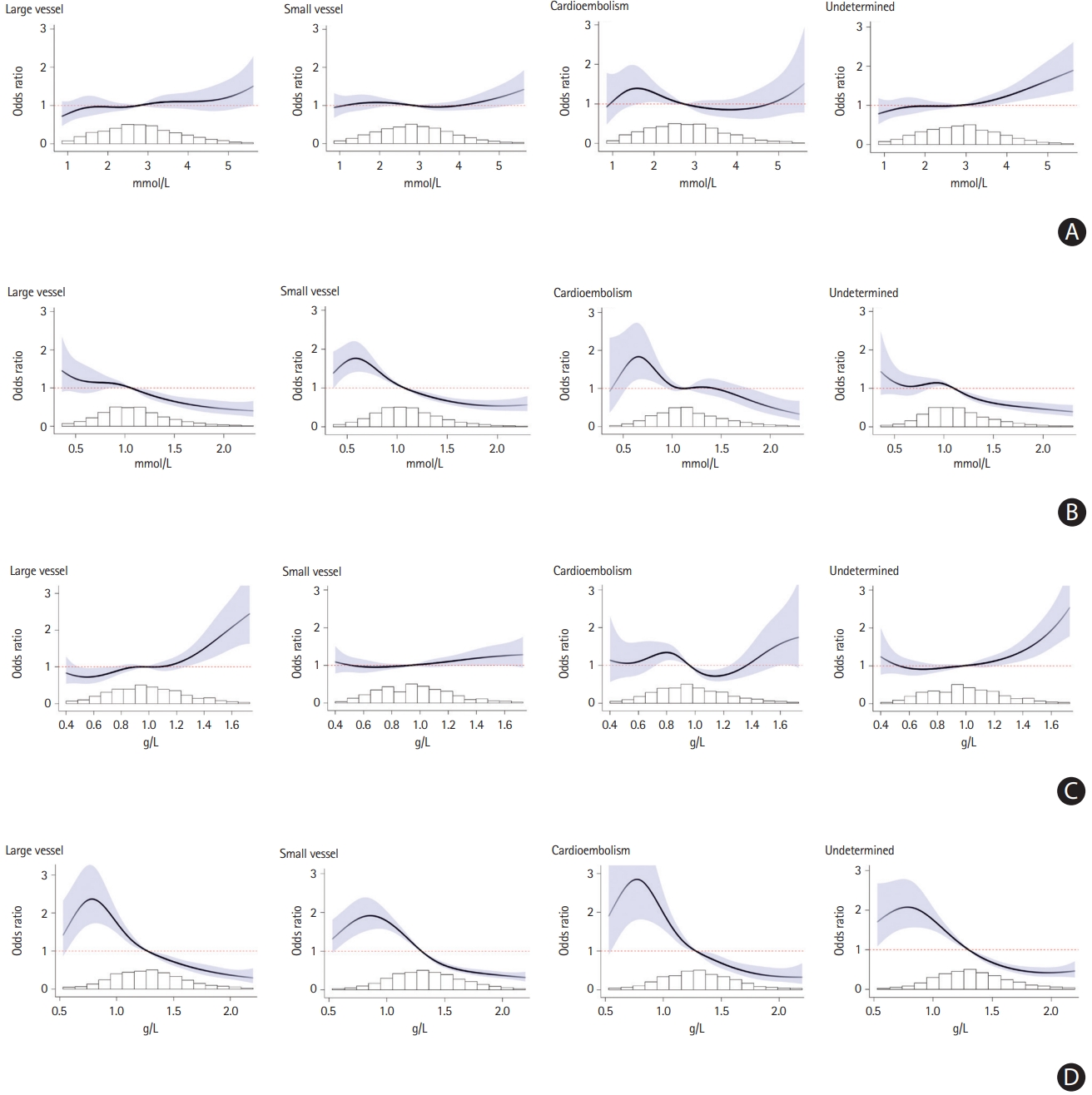
Association of (A) low-density lipoprotein cholesterol (LDL-C), (B) high-density lipoprotein cholesterol (HDL-C), (C) apolipoprotein B (apoB), (D) apoA1 by ischemic stroke subtype. Restricted cubic spline plot of association of (A) LDL-C (X-axis), (B) HDL-C (X-axis), (C) apoB (X-axis), (D) apoA1 (X-axis) with ischemic stroke subtypes. Spline curve truncated at highest and lowest 2% of values. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, pre-admission statin, and alcohol intake).
Apolipoproteins and stroke
Increasing apoB was associated with an increase in risk of ischemic stroke (OR, 1.10; 95% CI, 1.06 to 1.14), with an apparent threshold effect over approximately 1.0 g/L and associated with a reduced risk of ICH (OR, 0.88; 95% CI, 0.83 to 0.93 per SD) (Figures 1 and 2B). ApoB was a stronger predictor of ischemic stroke than LDL-C (P<0.0001) or non-HDL-C (P<0.0001). Increasing apoA1 was associated with a lower risk of both ischemic stroke (OR, 0.63; 95% CI, 0.61 to 0.66 per SD) and ICH (OR, 0.80; 95% CI, 0.75 to 0.85 per SD), and did not have significantly stronger association than HDL-C (Figures 1 and 2B). Within ischemic stroke subtypes, higher levels of apoB were associated with a higher risk of large vessel and undetermined etiology, but not significantly associated with small vessel or cardioembolism (Figure 1). ApoA1 was associated a lower risk of all ischemic stroke subtypes (Table 1 and Figure 3D).
Lipoprotein ratio, apolipoprotein ratio, and stroke
Increasing LDL-C/HDL-C ratio was associated with a higher risk of ischemic stroke (OR, 1.26; 95% CI, 1.21 to 1.31 per SD) and a lower risk of ICH (OR, 0.83; 95% CI, 0.78 to 0.88 per SD). The apoB/A1 ratio was associated with a higher risk of ischemic stroke (OR, 1.38; 95% CI, 1.32 to 1.44) but was not significantly associated with ICH (OR, 1.00; 95% CI, 0.94 to 1.06 per SD) (Figure 1). The ratio of apoB/A1 had a stronger magnitude of association with ischemic stroke than the LDL-C/HDL-C ratio (P<0.0001) or non-HDL-C/HDL-C ratio (P<0.0001), and was consistent among ethnicities (Tables 1 and 2). The PAR for the apoB/A1 ratio was numerically largest for ischemic stroke caused by large vessel disease (47.6%; 95% CI, 40.1% to 55.2%) (Table 2 and Figure 1). The PAR for the apoB/A1 ratio was more related to the magnitude of association of apoA1 than apoB with ischemic stroke.
Supplementary Figure 3 reports the association of LDL-C/ApoB and HDL-C/ApoA1 ratio with ischemic stroke and ICH, with LDL-C/ApoB associated with a reduced risk of ischemic stroke and HDL-C/apoB associated with an increased risk of ICH (Supplementary Figure 3). Splines for the association of apoB and apoA1 with ischemic stroke by age are reported in Supplementary Figure 4.
Analysis by region
The pattern of association of lipoproteins and apolipoproteins with ischemic stroke was generally consistent among regions (Supplementary Figures 5 and 6). We found differing patterns of association by region for ICH. Increasing LDL-C and apoB were associated with a lower risk of ICH in China, but there was no association in South Asia, and an association with a higher risk of ICH in other regions. Increasing HDL-C was associated with an increased risk of ICH in all regions (Supplementary Figure 7). Increasing apoA1 was associated with a reduced risk of ICH in China, and all regions other than South Asia, where the risk was increased (Supplementary Figure 8).
Analysis by prior statin use
We report no difference in magnitude of association between apoB/A1 ratio and odds of ischemic stroke in those reporting pre-admission statin use (OR, 1.50; 95% CI, 1.12 to 1.99 and OR, 2.53; 95% CI, 1.85 to 3.45 for tertile 2 and 3 vs. tertile 1) and those not reporting pre-admission statin use (OR, 1.31; 95% CI, 1.16 to 1.48 and OR, 1.93; 95% CI, 1.71 to 2.18 for tertile 2 and 3 vs. tertile 1).
Discussion
In the INTERSTROKE study, the risk of first-ever ischemic stroke was associated with concentrations of apolipoproteins and lipoproteins. However, the magnitude and pattern of the associations differed amongst different etiological subtypes, with the strongest association of apoB reported for large vessel ischemic stroke, and the weakest for cardioembolic stroke. The ratio of apoB/A1 was associated with a higher OR for risk of ischemic stroke than LDL-C/HDL-C ratio overall, and consistent among different ethnicities. A reversed association of LDL-C, HDL-C, and apoB with ICH, compared to ischemic stroke was identified, but the observations for ICH were inconsistent among different regions. Increasing levels of apoA1 were associated with both a lower risk of ICH, and ischemic stroke, and the only lipid fraction to have a generally consistent association for ischemic and hemorrhagic stroke.
We previously reported that apoB/A1 ratio was associated with 34% of the PAR for ischemic stroke, which is lower than the PAR reported for myocardial infarction in INTERHEART (49%). Ischemic stroke is more etiologically heterogenous than AMI, where large vessel atherosclerosis is the predominant etiology for AMI but accounts for only about 20% of ischemic stroke etiologies. Unlike AMI, where a linear increase in risk is reported with apoB, the association of apoB with ischemic stroke is curvilinear, with an increased risk only becoming evident with apoB levels over 1 g/L, a finding that supports an important role in pathogenesis of ischemic stroke, though less important than for AMI. Within ischemic stroke subtypes, we observed variations in the magnitude of association of apoB and LDL by stroke etiology. The association of apoB and ischemic stroke was strongest for large vessel (PAR of 13.4%) and stroke of undetermined etiology (PAR 7.1%) (Figure 3C). However, the PAR we report for the association of apoB/A1 ratio for large vessel disease (47.6%) is similar to that reported for AMI in INTERHEART (49%), and apoA1 was a greater contributor to PAR than apoB. We found no significant association of apoB with cardioembolic stroke. This is not unexpected, as the predominant etiology for cardioembolic stroke was atrial fibrillation, which is not known to have an association with LDL-C or apoB. We found a similar association of apoB with both large vessel and ‘cryptogenic’ ischemic stroke, which suggest that large vessel disease, rather than covert atrial fibrillation, is a more likely etiology in patients with ‘unexplained’ ischemic stroke and may reflect the clinical importance of atherosclerotic disease of <50% stenosis. In a recent Mendelian randomization study, genetically elevated LDL-C was associated with an increase in large vessel ischemic stroke, but not small vessel or cardioembolism [39].
Differences in case mix of ischemic stroke subtypes might explain inconsistent findings in prior studies [8]. For example, in our study, the magnitude of association of apoB with all ischemic stroke in China was lower than for Europe and North America, but small vessel disease accounted for 60% of the stroke subtype in China. Nevertheless, when we compared the association within ischemic stroke subtypes, the patterns of association are similar among regions. These findings are also consistent with some observations from secondary prevention trials of statin therapy. In the Japan Statin Treatment Against Recurrent Stroke (J-STARS) trial [40] (n=1,578) in Japan, low dose pravastatin did not reduce the overall risk of recurrent ischemic stroke, but, in post hoc analyses, did reduce the risk of recurrent large vessel ischemic stroke. In the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial [41] (n=4,731), atorvastatin 80 mg significantly lowered the risk of recurrent ischemic stroke, with a risk reduction of 0.63 (0.46 to 0.87) in those with large vessel disease and 0.89 (0.70 to 1.13) in those with small vessel disease. In the recent trial by Amarenco et al. [42], an LDL target of less than 1.8 mmol/L was more effective than an LDL target of 2.3 to 2.6 mmol/L for secondary stroke prevention in patients with atherosclerotic large vessel disease. Overall, however, statin therapy reduces the risk of ischemic stroke, with a similar relative risk reduction in AMI in individuals at increased cardiovascular risk [43].
Both apoB and apoA1, and their ratio, had a larger magnitude of association with ischemic stroke than did lipoprotein cholesterol levels, and their ratios. These findings are consistent with those reported for AMI in the INTERHEART study [37], for ischemic stroke in the Apolipoprotein MOrtality RISk (AMORIS) study [23] and for recurrent stroke following transient ischemic attack in the Oxfordshire stroke study [44]. By contrast, other studies, including the Emerging Risk Factor Collaboration, reported a similar strength of association of lipoproteins and apolipoproteins with ischemic stroke. The INTERSTROKE and INTERHEART studies were conducted in ethnically diverse populations, while other large studies have been primarily confined to Europe and North America. In INTERSTROKE, the difference in PAR% of apolipoproteins over lipoproteins for stroke risk prediction was least prominent in North America and Europe (Table 2).
Consistent with reports from other epidemiological studies, we found an inverse association of total cholesterol and LDL-C with ICH, while higher levels of HDL-C were associated with a higher risk, although some prior studies report a null association [9,45]. The underlying pathophysiology to explain these observations is poorly understood, and may relate to the importance of lipoproteins in small vessel structure, as an inverse association of LDL-C with burden of white matter hyperintensities and microbleeds have been reported in some epidemiologic studies [46,47]. Furthermore, some individual trials (e.g., SPARCL trial [41]) have reported an increase risk of ICH with statin therapy, although meta-analyses of statin trials do not report an increased risk of ICH with statin use [41,48]. A Mendelian randomization study of UK-Biobank study reported an increased risk of ICH associated with HDL-related genetic variants [49]. While our findings for ischemic stroke were generally consistent by region, we found significant heterogeneity by region in both the magnitude and direction of association of lipoproteins with ICH (Supplementary Figure 8). These inconsistent findings by region may be due to chance, to differences in causal mechanisms for ICH, or differential effect of competing causes of stroke, particularly hypertension. For example, ICH may be a consequence of amyloid angiopathic small vessels, related to either small vessel ischemia (implicating increased apoB) or if apoB is important for maintaining small vessel integrity, lower levels may increase ICH risk. However, these considerations are merely speculative, and require further research, especially genetic studies employing Mendelian randomization approaches. Arguing against a causative role for HDL-C are studies reporting the absence of a cause-specific association of HDL-C with mortality, and complex associations with sociodemographic and lifestyle risk factors [50].
The only lipid fraction with a consistent association across all stroke subtypes was apoA1, which was associated with a lower risk of both ICH and ischemic stroke, and each of the etiological ischemic stroke subtypes. Unlike HDL-C, apoA1 has a number of functions [51], and low apoA1 levels have been associated with a higher risk of amyloid angiopathy [52]. ApoA1 also stimulates cholesteryl ester transfer protein (CETP) and apoE secretion from lipid-loaded macrophages, which may be a mechanism through which it exerts a reduced ICH risk [47]. In a recent genetic study, candidate-gene CEPT variants that reduce CEPT concentrations were associated with an increased risk of ICH [53]. An additional contributor may be the ratio of HDL-C to apoA1, where studies have reported this ratio may affect HDL-C function and increasing HDL-C/apoA1 ratio has been associated with an increased risk of cardiovascular disease, cancer, and death [54]. Our analyses of HDL-C/apoA1 ratio demonstrated a marked linear reduction in ICH risk with an increasing ratio, raising the speculative contention that this ratio may be an important determinant of ICH risk. However, the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial [55], in which apoA1 was increased by 22% was not associated with a lower risk of stroke, although participants with the largest increases in apoA1 had lower event rates.
In addition to known limitations inherent in case controls studies (e.g., recall bias, social desirability bias), our study has specific limitation relevant to the current analyses. We relied on clinical assessment for determination of ischemic stroke subtypes, as most cases did not undergo etiologic diagnostic testing, including vascular imaging. At the outset of the INTERSTROKE study, we appreciated the limited availability of a variety of diagnostic modalities and elected to have the stroke physician complete the case report form, for their assessment of most probable etiological source of ischemic stroke, based on clinical assessment when diagnostic tests were unavailable. However, this may be associated with misclassification of ischemic stroke subtype, which can be challenging, even in centers with routine access to etiological diagnostic testing, since common ischemic stroke etiologies share risk factors, and commonly co-exist. Therefore, we cannot draw definitive conclusions by ischemic stroke subtype. However, this source of bias should diminish, rather than amplify, our ability to detect differences among ischemic stroke subtypes. The case-control design incurred a potential effect of acute phase measurement, which may have also differed by stroke subtype, which is associated with stroke severity. However, in INTERSTROKE, we targeted recruitment of cases shortly after admission to hospital, to reduce this source of bias. Acute phase may have affected levels of lipoproteins in cases, and may contribute to a lower OR reported in our study compared to prospective cohorts studies for the association of LDL-C and risk of ischemic stroke. Alternatively, some [7,56], but not all [57] prospective cohort studies have reported a diminution of risk magnitude with increasing age, which might also be a reason for differences, as our study population was older than most inception cohorts. In 12% of our population, we did not get samples for lipid measurement, as collection of bloods was not practical in two countries included in INTERSTROKE, which may reduce generalisability of our findings, but is not expected to introduce bias as samples were missing in cases and controls from these sites.
Conclusions
The association of cholesterol, lipoproteins, and apolipoproteins with stroke varied by stroke subtype and etiological mechanism. The ratio of apoB/A1 was a better predictor of risk of ischemic stroke than LDL-C/HDL-C ratio. Population-level interventions to reduce LDL-C or apoB, or introduce statin therapy in intermediate and high cardiovascular risk populations,57 are expected to have a major impact on the global burden of stroke, but with differing magnitudes of effect on stroke subtypes.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2021.02152.
Exclusion criteria for cases
Guidance to sites for selection of controls
Demographic and clinical characteristics of cases
Mean concentration of lipids, lipoproteins, apolipoproteins, and their ratios in controls (men and women) by region
Adjusted mean concentration of lipids, lipoproteins, apolipoproteins, and their ratios in cases and controls
Association of total cholesterol with (A) ischemic stroke and (B) intracerebral hemorrhage. Restricted cubic spline plots of association of total cholesterol (X-axis) with ischemic stroke and intracerebral hemorrhage. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, and alcohol intake).
Association of total cholesterol by ischemic stroke subtype. (A) Large vessel, (B) small vessel, (C) cardioembolism, (D) undetermined. Restricted cubic spline plot of association of total cholesterol (X-axis) with ischemic stroke subtypes. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, and alcohol intake).
Association of lipoprotein/apolipoproteins ratio with ischemic stroke and intracerebral hemorrhage. (A) Ischemic stroke (low-density lipoprotein cholesterol [LDL-C]/apolipoprotein B [apoB]), (B) ischemic stroke (high-density lipoprotein cholesterol [HDL-C]/apoA1), (C) intracerebral hemorrhage (LDL-C/apoB), (D) intracerebral hemorrhage (HDL-C/apoA1). Restricted cubic spline plot of association of apolipoproteins (ApoB and ApoA1) (X-axis) with ischemic stroke, by age cut-point. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, and alcohol intake).
Association of (A) apolipoprotein B (apoB) and (B) apoA1 and ischemic stroke subtype by age. Restricted cubic spline plot of association of lipoproteins (low-density lipoprotein cholesterol and high-density lipoprotein cholesterol) (X-axis) with ischemic stroke by China, India, and other regions of the world. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, and alcohol intake).
Association of lipoproteins with ischemic stroke (China, India, and all other regions). Restricted cubic spline plot of association of lipoproteins (A: low-density lipoprotein cholesterol [LDL-C]; B: high-density lipoprotein cholesterol [HDL-C]) (X-axis) with intracerebral hemorrhage by China, India, and other regions of the world. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-tohip ratio, and alcohol intake).
Association of apolipoproteins with ischemic stroke (China, India, and all other regions). Restricted cubic spline plot of association of apolipoproteins (A: apoB; B: apoA1) (X-axis) with ischemic stroke by China, India, and other regions of the world. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, and alcohol intake).
Association of lipoproteins and intracerebral hemorrhage (ICH) (China, India, and all other regions). Restricted cubic spline plot of association of apolipoproteins (A: low-density lipoprotein cholesterol [LDL-C]; B: high-density lipoprotein cholesterol [HDL-C]) (X-axis) with ICH by China, India, and other regions of the world. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-tohip ratio, and alcohol intake).
Association of apolipoproteins with intracerebral hemorrhage (ICH) (China, India, and all other regions). Restricted cubic spline plot of association of (A) apoB and (B) apoA1 (X-axis) with ischemic stroke and ICH. Spline curves truncated at highest and lowest 2% of values. Light blue shading denotes 95% confidence interval. All splines adjusted for age, sex, geographic region, and potential confounders (smoking, diabetes mellitus, physical activity, diet, psychosocial factors, waist-to-hip ratio, and alcohol intake).
Notes
Disclosure
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Vastra Gotaland (Sweden), and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MSD, Swedish Heart and Lung Foundation, Chest, Heart and Stroke Scotland, and The Stroke Association, with support from The UK-Stroke Research Network. The sponsors had no role in data collection, analyses or the decision to submit for publications.
Graeme J. Hankey reports personal fees from Bayer and Medscape, outside of the submitted work. All other authors declare no competing interests.
References
Appendices
INTERSTROKE project office staff, national coordinators, investigators and key staff
jos-2021-02152-app1.pdfINTERSTROKE (methods, risk factor measurement)
jos-2021-02152-app2.pdf

