Predictors of Therapy Response in Chronic Aphasia: Building a Foundation for Personalized Aphasia Therapy
Article information
Abstract
Chronic aphasia, a devastating impairment of language, affects up to a third of stroke survivors. Speech and language therapy has consistently been shown to improve language function in prior clinical trials, but few clinicially applicable predictors of individual therapy response have been identified to date. Consequently, clinicians struggle substantially with prognostication in the clinical management of aphasia. A rising prevalence of aphasia, in particular in younger populations, has emphasized the increasing demand for a personalized approach to aphasia therapy, that is, therapy aimed at maximizing language recovery of each individual with reference to evidence-based clinical recommendations. In this narrative review, we discuss the current state of the literature with respect to commonly studied predictors of therapy response in aphasia. In particular, we focus our discussion on biographical, neuropsychological, and neurobiological predictors, and emphasize limitations of the literature, summarize consistent findings, and consider how the research field can better support the development of personalized aphasia therapy. In conclusion, a review of the literature indicates that future research efforts should aim to recruit larger samples of people with aphasia, including by establishing multisite aphasia research centers.
Introduction
Aphasia is a devastating language disorder most commonly resulting from a cortical lesion to the perisylvian region of the language-dominant hemisphere [1,2]. Despite a general lack of public knowledge [3], chronic aphasia (≥6 months post-onset) is not an uncommon disorder, affecting up to a third of stroke survivors [4]. Behavioral speech and language therapy (SLT) has been shown to be an efficacious approach to improve language function in persons with aphasia (PWA) as a group [5,6], and remains a mainstay in the clinical management of aphasia [7]. Nonetheless, there is notorious and unexplained heterogeneity in therapeutic effects at the individual level [8-10]. Critically, prior efforts to identify individual predictors of therapy response [11-13] have not yet managed to reliably answer two fundamental questions: (1) who responds to SLT and (2) to what degree? Meanwhile, clinicians who routinely administer SLT struggle with personalized therapy planning and prognostication [14].
More specifically, clinicians are presently unable to determine with a consistent degree of confidence whether and to what degree any given PWA will respond to therapy based on pre-therapy individual characteristics. To this end, addressing questions (1) and (2) above is a crucial prerequisite to facilitate the development of personalized aphasia therapy (PAT), i.e., therapy tailored to maximize each individual’s potential language recovery with reference to evidence-based clinical guidelines.
Innovative technological developments and advances in therapeutic approaches in the literature have increasingly emphasized the pressing demand for personalized solutions in aphasia therapy [15,16]. However, few generalizable and clinically applicable predictors of therapy response have been identified to date. The primary cause likely stems from the fact that the relevant literature is predominated with single-subject and small group studies [13,17]. While single-subject and small group study designs are well-suited to acquire detailed accounts of the experiences of individuals with specific characteristics, they are, by definition, not intended to reflect population parameters. From a statistical perspective, the issue of individual variability in therapy response is substantially exacerbated due to small sample sizes, which leaves most prior studies with severely limited statistical power to detect causal relationships that can be leveraged to guide PAT at the population level [18,19]. Epidemiological trends indicating a rising prevalence of aphasia [20] and, as a result, a growing societal burden of aphasia [21], have rendered research aiming to improve clinical management of aphasia an immediate public health priority.
In the interest of facilitating the development of PAT, this narrative review offers a comprehensive overview of the current state of the literature on personalized predictors of therapy response in chronic aphasia. We restrict our focus to chronic aphasia as recent large-scale randomized controlled trials have failed to demonstrate robust therapeutic effects beyond spontaneous recovery when therapy was commenced in the acute (≤2 weeks of stroke onset) phase of recovery [22-24]. Specifically, we emphasize recent studies that have explicitly examined the association between common biographical, neuropsychological, or neurobiological variables, and response to impairment-based SLT. Given the limitations of the literature, indirect evidence, such as cross-sectional studies, studies of spontaneous aphasia recovery, and other derived sources are similarly considered as applicable. We conclude by discussing how clinical prognostication can be improved through future research endeavors.
Predictors of treated recovery in aphasia
There is an unequivocal and obvious benefit of identifying robust predictors of therapy response in aphasia. Nevertheless, few comprehensive studies have been carried out in pursuit of this goal. One potential reason is the cost of conducting large-scale therapy studies in aphasia. The cost associated with participant recruitment, administration of therapy, collection of an extensive dataset, and multiple magnetic resonance imaging (MRI) scanning sessions per participant is high, and accomplishing studies of this scale requires both an interdisciplinary team of professionals and tangible resources. Notwithstanding, a few studies have directly examined predictors of therapeutic effects in samples of varying sizes [25]. The evidence amassed through these studies is discussed in what follows.
Biographical predictors
Age
The brain’s capacity for cognitive processing decreases gradually in normal aging [26]. Granted that language restoration relies on functional and/or structural plasticity and numerous findings showing a steady decrease in brain plasticity with age [27,28], intuition might suggest that older individuals are less likely to show favorable language recovery. In line with this view, several studies have suggested that younger age might mediate positive outcomes in the acute recovery phase [29-35]. For instance, Ali et al. [29] (2021) found younger age (<55 years) to be the strongest predictor of recovery in a large sample of PWA. However, this finding has not been consistently replicated in similar studies [36-40]. One potential reason for this discrepancy is that the relationship between age and recovery may be confounded by stroke age; aphasia is more likely to emerge as a consequence of stroke in older individuals [39-42] and older individuals generally present with more severe aphasia (i.e., fluent aphasia as opposed to nonfluent) [42-45].
Several aphasia therapy studies have observed greater therapy-induced improvements in younger compared to older individuals [46-50]. Recent research in our lab supports these findings. A retrospective study examining the effects of age on outcomes in several prior therapy studies conducted in our lab found a complex relationship between age and outcomes [51]. Johnson et al. [51] reported multicollinearity between age and variables such as sex and education, whereas the independent effects of age varied across studies. By contrast, a number of other aphasia therapy studies have failed to find a consistent relationship between age and therapy success [52-55]. Correspondingly, recent qualitative literature reviews have consistently described the relationship between age and language function in aphasia as equivocal [2,37,56]. Thus, while further research is warranted, any given study aiming to predict therapy response in aphasia should examine the effects of age on the known effect of age on brain repair mechanisms.
Sex
A long-held view that postulates sex differences in language processing has motivated research on the effects of sex on aphasia recovery [57,58]. In their seminal study, Shaywitz et al. [58] noted strongly left-lateralized frontal activation in males, whereas more diffuse neural systems were engaged in females bilaterally in response to a language task. Consistent with the notion that females have more flexibility to recover from left hemisphere (LH) stroke due to bilateral engagement, some studies have noted better recovery of language function in females [29,59,60]. However, most studies have not found differences in language recovery depending on sex [36-39,55,61]. Relatively better recovery in males has even been reported [32]. Therefore, at present, there is no conclusive evidence to suggest that sex is a determining factor of therapy-induced language recovery.
Handedness
Differences in language recovery based on handedness are grounded in theories of language lateralization, similar to theorized differences based on sex. Children born left-handed have been shown to exhibit more bilateral cortical representation of language compared to right-handed children (15%–33% vs. 7%–9%, respectively) [62], although comparably pronounced differences have not been observed in adults [62-64]. Prior studies have not provided conclusive support for the notion that left-handed individuals have greater capacity for language recovery due to greater bilateral representation of language [2,37,39].
Education
Research examining the relationship between education and language recovery draw from the idea that a greater degree of education may be an indication of relatively preserved cognitive reserve after brain damage. While few studies have directly assessed the predictive value of education for therapy outcomes, conflicting results have been reported regarding the effects of education on language recovery in general [31,65-68]. Connor et al. [69] and Hillis and Tippett [65] both reported an association between education and aphasia severity in the chronic phase of recovery, but not with extent of language recovery. The authors noted that education might serve as a proxy variable for a plethora of other factors such as discipline or determination, cognitive reserve, economic resources, healthy lifestyle, literacy level, socio-economic status, occupation, and access to healthcare [70]. Thus, it remains to be determined whether education independently influences response to therapy.
Time post-stroke
Time post-stroke (TPS) is an obvious and crucial determinant of early spontaneous recovery of language function, as the recovery trajectory is steepest in the 1st weeks through 6 to 12 months following stroke onset [39,59,71]. In the chronic phase, TPS does not seem to be related to response to therapy in PWA [53,54,72,73]. Moss and Nicholas [73] offered a comprehensive review of the effects of TPS on therapy outcomes in individuals beyond 1-year post-onset. In short, the authors found no correlation between TPS at which therapy was initiated with response to therapy [73].
Psychosocial factors
The literature on the effects of psychosocial factors such as social support and mood on therapy response is scarce, but a handful of studies have found that social support and mood contribute to individuals’ quality of life and sense of autonomy [74-76]. These findings have led some researchers to argue that strong social networks and motivation facilitate progress in therapy [16,77]. Consequently, functional communication and community support therapy approaches generally focus on strengthening social relationships and improving mood with the intention of enhancing individuals’ sense of autonomy. The Life Participation Approach to Aphasia (LPAA) philosophy, which aims to empower PWA to actively participate in their rehabilitation and to engage in daily activities of interest, is particularly relevant in this respect [78]. Group-based therapy approaches likewise aim to combat the social isolation commonly experienced by PWA [79-81]. Furthermore, various language therapy approaches have been found to improve mood in PWA [82-84]. Therefore, psychosocial factors may contribute to the likelihood of positive outcomes following therapy, although further research on the precise psychosocial predictors is necessary.
Aphasia type
Aphasia type is dependent on factors such as lesion size and location, and by extension higly correlated with aphasia severity. Nonetheless, aphasia classification has been investigated in relation to clinical outcomes [8,40,59]. In terms of therapy response, however, the independent predictive value of aphasia type remains unclear. Individuals with Broca’s aphasia may have positive prognosis in the 1st year following stroke [85], whereas in the chronic phase, individuals with fluent aphasia subtypes (e.g., anomic and conduction aphasia) have been shown to respond better to therapy than nonfluent individuals [8,86]. For example, Kristinsson et al. [8] observed a larger overall therapy effect in fluent compared to nonfluent individuals. These findings suggest that aphasia type can inform prognostication in aphasia therapy, although the potential confounding effects of aphasia severity, lesion size, and location need to be taken into consideration.
Summary of biographical predictors
In terms of biographical predictors, limited generalizable knowledge has accumulated in the literature. Prior studies suggest a complex and potentially confounding relationship between age and aphasia type with therapy success. Time since stroke-onset does not seem to negatively affect therapy response in chronic aphasia, and sex and handedness similarly do not seem to affect therapy response. On the other hand, social support and motivation are potentially favorable attributes, but further research where these predictors are better defined is needed. Similarly, the independent predictive value of education remains to be determined. In conclusion, systematic evaluation of biographical predictors of therapy outcomes is needed before this knowledge can be applied in clinical practice.
Neuropsychological predictors
Aphasia severity
The initial severity of language deficits is commonly recognized as the most consistent predictor of aphasia recovery [2,70]. Aphasia severity is strongly associated with spontaneous recovery, with increased rate of complete recovery observed in individuals with milder symptoms [33,34,39,66,67,87]. Correspondingly, aphasia severity is generally considered the single strongest predictor of response to impairment-based therapy [16,17,48,59]. Performance on confrontation naming tasks, as a proxy measure for aphasia severity, has similarly emerged as the strongest predictor of response to anomia therapy [12,55,88,89]. For instance, Seniów et al. [55] found that severity of anomia prior to therapy was highly correlated with all post-therapy language outcomes in a relatively large sample of 78 participants with chronic aphasia.
Despite the broad consensus in the literature, the precise nature of the relationship between aphasia severity and therapy outcome has yet to be fully mapped out. While some researchers have found a relative advantage for individuals with milder aphasia [52,88], others have observed an advantage for individuals with more severe aphasia [90], and some have reported mixed results [54]. Recent work from our lab offers a potential explanation for this discrepancy; we found that participants with a mild language impairment responded well to semantically-focused therapy, whereas those with more severe symptoms reponded better to phonologically-focused therapy [8]. Thus, aphasia severity not only determines general recovery after therapy, but also uniquely impacts response to different types of therapy.
Cognitive processing
Aphasia frequently co-occurs with deficits affecting other cognitive domains [12,55,91-95]. However, there is considerable heterogeneity in terms of both the degree of cognitive deficits and the specific cognitive domains affected across individuals [96]. Several studies have examined the role of non-linguistic cognitive impairments in language therapy in chronic aphasia [12,55,97,98]. Briefly, all of the referenced studies found cognition at baseline to be associated with therapy success. Seniów et al. [55] reported that visuo-spatial working memory predicted improvement in both naming and comprehension; Lambon Ralph et al. [12] found that a principal component analysis-derived ‘cognitive’ factor was able to predict both immediate and longer-term therapy gain; Dignam et al. [97] found that verbal short-term memory ability significantly predicted naming gains for treated items immediately after therapy and for untreated items immediately after therapy and at a 1-month follow-up testing; and, last, participants in Fillingham et al. [98] study who responded well to therapy had better recognition memory, executive/problem solving skills, and monitoring ability compared with nonresponders.
Premorbid intelligence
Premorbid intelligence was proposed as a potential prognostic factor for aphasia recovery in early studies [99]. Kertesz and McCabe [99] found that performance on a non-verbal intelligence task was dependent on aphasia type in that individuals with poor comprehension (i.e., global, Wernicke’s, and transcortical sensory aphasias) performed poorly, whereas performance of individuals with Broca’s, transcortical motor, conduction, and anomic aphasia was comparable to that of non-aphasic controls. Others have similarly found a relationship between premorbid intelligence and aphasia severity [100], but not with aphasia recovery [100,101]. Therefore, although there may be an association between measures of intelligence and aphasia severity, recovery of language function does not seem to depend on premorbid intelligence. It is important to note that measuring intelligence in PWA is inherently a difficult task and precision can easily be compromised. Non-verbal tasks must be utilized to bypass any confounding effects of language deficits, but as most such tasks necessarily entail some degree of verbal instructions, even non-verbal intelligence measures may not accurately reflect intelligence in PWA.
Summary of neuropsychological predictors
In conclusion, severity of aphasia is a driving predictor of therapy success; this association is robust and independent. The precise nature of the relationship, e.g., whether severity holds greater predictive value in milder or more severe individuals remains to be thoroughly studied. Variable cognitive factors have been suggested to mediate therapy response as well. It is unclear whether the contribution of different cognitive factors is independent of other cognitive factors, and whether these factors are confounded by aphasia severity. Given the heterogeneity of cognitive abilities in PWA and the variable cognitive domains that have been linked to recovery, further research will be required to address this question.
Neurobiological predictors
The neurobiological basis of aphasia has attracted a great deal of research attention, dating back to the seminal works of Paul Broca [102,103] and Carl Wernicke [104]. Consequently, a vast literature on the neural bases of aphasia and aphasia recovery has accumulated. Most studies have investigated cross-sectional brain-behavior relationships or spontaneous recovery, but recent studies have increasingly focused on therapy-induced neuroplastic changes and neural predictors of therapy response [11,53].
Lesion size
The sheer extent of lesion damage is a critical determinant for post-stroke neural repair mechanisms. Larger lesions inevitably leave less volume of intact brain tissue available for remapping and reorganization of language [105]. Furthermore, larger lesions are unavoidably more likely to impact a greater number of language network nodes, as well as domain-general systems supporting language processing [106]. Correspondingly, lesion volume has consistently been found to be inversely related to spontaneous language recovery [106-114] and chronic (>6 months post-onset) aphasia severity [115-117].
In terms of therapy studies, few studies have explicitly predicted language outcomes from lesion volume alone [107]; however, therapeutic effects are generally thought to depend on lesion size based on neurobiological principles [25]. Following this rationale, multiple studies have included lesion size as a covariate in analyses predicting outcomes of language therapy [118-121]. For example, Fridriksson [119] localized brain damage associated with poor response to anomia therapy in 26 participants by adjusting for the effect of lesion size. These studies assume lesion size explains a certain amount of variability in the respective outcomes and that accounting for this variability will increase the power of the analysis to detect an effect of interest.
A handful of studies have failed to identify a relationship between lesion size and treated recovery [121,122]. Although it may be reasonable to conclude that larger lesions deter recovery, lesion location can be equally important in predicting language outcomes [113,123,124]. Thus, the relationship between lesion volume and treated recovery is not entirely independent or linear in nature [124-126]. Instead, the predictive value of lesion size should be considered in lieau with other lesion characteristics (e.g., location).
Lesion location
Consistent with the network organization of language in the brain [1], some regions of the brain serve as integral communication hubs (e.g., posterior middle temporal gyrus [MTG] [127-129]). Damage to network hubs that mediate communication between multiple brain regions has greater negative impact on the extent of language impairment and language recovery than damage to non-hub regions. The detrimental effect of damage to network hubs for early language recovery was studied in detail in the 1980s and 1990s. In particular, damage to the temporoparietal junction, encompassing posterior temporal and inferior parietal regions, was consistently associated with poor recovery [107,112,114,130-133]. More recent work has largely confirmed these findings [31,67,134,135]. Hillis et al. [31] investigated lesion characteristics that predicted naming outcome in a combined sample of 251 participants. The findings revealed a double dissociation; greater lesion load in the LH posterior superior temporal gyrus and superior longitudinal fasciculus/arcuate fasciculus (SLF/AF) was associated with poorer recovery, whereas preservation of these regions was associated with good recovery of naming [31].
Correspondingly, damage to similar hub regions of the language network has been associated with treated recovery [11,118,119,125,136]. For instance, Fridriksson [119] found that damage to the posterior portion of the LH MTG and temporal-occipital junction had a particularly negative effect on language outcomes following anomia therapy. Other studies have reported an association between sparing of tissue in the temporoparietal junction and positive therapy outcome [11,118,125]. Furthermore, Parkinson et al. [125] showed that individuals with relatively greater lesion load in anterior regions and the basal ganglia intact responded better to therapy than their counterparts with relatively larger lesions in posterior regions.
Consistent with findings suggesting that there is no guarantee that all individuals respond equally well to all therapy protocols [8], lesion location predicting therapy response may depend on specifics of the therapy protocol. For example, contrary to findings linking damage to frontal regions (including Broca’s area) with poor therapy outcomes [122], Fridriksson et al. [136] found that the fluency-inducing effect of speech entrainment (SE) therapy, an intervention that relies on mimicking audiovisual speech in real time, was associated with lesions to inferior and middle frontal gyri. According to the authors, this finding indicates that SE compensates for damage to speech production mechanisms located in the inferior frotal gyrus (IFG), provided that alternative neural pathways are still intact to support the function [136].
The findings summarized here indicate that damage localized in or around the temporoparietal junction is strongly associated with therapy-induced recovery. Equally importantly, the findings discussed above also highlight that there is not a oneto-one correspondence between damage in a given region and prognosis. As an example, damage to Broca’s area has both been found to facilitate therapy success [125,136] and to negate therapy success [122]. The complex nature of the interacting relationship between lesion location, lesion size, and therapy response has motivated researchers to increasingly adopt measures of functional and structural integrity of intact brain tissue to study aphasia recovery. Based on the notion that neuroplasticity relies on intact brain tissue, the rationale underlying these studies can simply be delineated as such; neural factors beyond frank lesion damage may provide alternative and complementary means to explain aphasia recovery.
Cortical activity
A number of studies have investigated the neural mechanisms underlying performance on specific language tasks in cross-sectional designs [137,138]; the normal course of spontaneous recovery [134,139], and therapy-induced changes in neural activity [11,53]. However, the literature to date offers limited examples of baseline functional activity measures used directly to predict therapy outcomes [119,122]. As a result, the neural substrates of treated language recovery remain elusive. Notwithstanding, several mechanisms underlying language reorganization have been proposed based on direct and indirect empirical evidence [13,19,140-145].
Seminal paper of Saur et al. [139] is frequently referenced as a cornerstone study in this literature. Briefly, findings of Saur et al. [139] have been interpreted as evidence for the dynamic and time-dependent reorganization of language following stroke, incorporating functional recruitment of both the LH and the right hemisphere (RH) at different timepoints. These findings manifest, in some ways, a persistent debate surrounding the role of the RH in language recovery [146]. Heiss and Thiel [146] proposed that optimal recovery relies on normalization of activity in intact LH language regions, whereas extensive RH activation might be maladaptive for successful recovery. Subsequent studies have elaborated on Heiss and Thiel’s framework in several important ways. First, if lesion damage is relatively small and/or focally located, reorganization seems to mainly occur in intact premorbid language regions and perilesional areas of the LH. Such ‘normalization’ or re-recruitment of language regions is typically associated with favorable spontaneous or therapy-induced recovery [19,119,122,141,147-154]. Second, in individuals whose lesion covers a large region within the LH or disproportionally affects critical hub regions, recruitment of RH regions has been suggested to facilitate recovery to a certain degree in some individuals, even though the compensatory effect of the RH may be restricted [142,146,155-160]. Third, brain regions available for reorganization recruitment generally fulfill the following criteria [161]: (1) they comprise lesion homologue regions in the RH and/or perilesional regions in the LH [144,160,162]; (2) they had the potential to subserve language functions prior to stroke (i.e., ‘redundant’ activation), as opposed to a takeover by brain regions unrelated to language processing (i.e., ‘vicarious’ activation) [19,145]; and (3) they may have been affected by dynamic diaschisis [163] and/or by inhibitory ipsilateral and contralateral influences [146].
In terms of therapy research, several important findings have been reported. Therapy gains have consistently been associated with recruitment of language regions in the LH [11,164-169] or perilesional activation [11,170]. Nonetheless, multiple studies have reported a relationship between therapy gains and recruitment of RH lesion homologue regions [159,171,172] or bilateral activation [53,166,173-177]. Furthermore, some researchers have emphasized that recruitment of domain general networks not specific to language may facilitate recovery in some individuals [137,171,178].
Only a handful of studies have aimed to directly predict therapy response from baseline functional activity or activity change pre- to post-therapy [11,117,119,122,179,180]. Marcotte et al. [122] found that baseline activation in LH precentral gyrus and recruitment of LH inferior parietal lobule predicted response to semantic feature analysis (SFA) anomia therapy in nine participants with chronic aphasia. Applying a similar therapy protocol, van Hees et al. [177] found that pre-therapy activity in the LH caudate nucleus predicted therapy success in eight participants following SFA therapy, whereas recruitment of the LH supramarginal gyrus and RH precuneus correlated with response to Phonological Components Analysis [181] therapy. Fridriksson [119] found that improved naming performance was predicted by increased activity in both anterior and posterior regions of the LH. A follow-up study by Fridriksson et al. [11] reported that change in activation in perilesional regions in the frontal lobe was a strong predictor of therapy response in 30 participants, whereas baseline activity was less informative. Menke et al. [180] predicted short-term therapy gain from activity change bilaterally in the hippocampal formation, RH precuneus and cingulate gyrus, and bilaterally in the fusiform gyrus. By contrast, long-term therapy success was predicted by recruitment of the RH Wernicke’s homologue and perilesional regions in the LH temporal lobe [180]. Last, Abel et al. [179] observed the strongest predictive value within language regions of the LH in a sample of 14 participants, but neuroplastic recovery processes were somewhat dependent on individuals’ deficit profiles (i.e., whether individuals had primarily semantic or phonological deficits).
Lesion profiles have similarly stifled attempts to inform the neural reorganization of language. In particular, the heterogeneity of lesion extent and location presents a major challenge for functional MRI (fMRI) studies in aphasia [19,140,182]. Briefly, a sizable literature suggests that activity patterns associated with language recovery are highly dependent on both lesion size and location of lesion damage [161,119,122,148,157,166,168,183-186]. Thus, future studies subserving the purpose of improving clinical prognostication should investigate activation patterns in tandem with other lesion characteristics.
In summary, despite the large body of literature that has accumulated over the past few decades, the extent to which treated recovery is predictable from baseline task-based functional activity remains unclear. In the interim, the empirical evidence indicates that the degree of language recovery relies to a great extent on reorganization within residual language networks, although precise activation patterns are likely determined by lesion characteristics. Importantly, many of the findings reported in the literature are difficult to interpret and generalize to other study samples due to small sample sizes. Only 7/32 studies in Schevenels et al. [13] recent review included a sample size of n>10 and only two studies recruited more than 20 participants. As the statistical power to detect brain-behavior relationships may be severely reduced in samples with n<30 [18], the findings discussed herein must be interpreted with caution. Therefore, in order to illuminate the contribution of functional activity for the prediction of treated recovery in aphasia, future studies will additionally need to rely on substantially larger study samples.
Functional connectivity
Researchers have increasingly taken advantage of functional connectivity measures (e.g., resting-state functional fMRI [rsfMRI]) to characterize functional network integrity in aphasia. Unlike task-based fMRI, rsfMRI does not require the individual to perform a task in the MRI scanner. The obvious benefit of bypassing language deficits has led to an increase in the number of publications utilizing rsfMRI in the aphasia literature. As a group, PWA show hypoconnectivity across multiple resting-state networks [187-189] and connectivity strength in some of these networks correlates with specific language functions [137,178,188,189,190-193]. Similar to task-based activation, connectivity strength is largely determined by lesion characteristics, with increased RH connectivity correlated with larger lesions [193]. Importantly, gradual normalization of integration within (e.g., the semantic network [187]) and segregation across brain networks correlates with language recovery [188]. Correspondingly, therapy success is characterized by normalization of functional connectivity [175,177,194-198] with increased connectivity of the LH IFG emerging as a common denominator among recovered individuals [175].
In one of the first attempts to predict therapy response from functional connectivity data, Marcotte et al. [196] studied connectivity changes in the default-mode network (DMN) in eight participants with aphasia who underwent SFA therapy. Therapy elicited improved integration in the posterior area of the DMN concurrent with language improvement [196]. Van Hees et al. [177] found that connectivity (amplitude of low frequency fluctuations) in the RH MTG correlated with response to phonological anomia therapy in eight chronic individuals, with a shift to increased temporoparietal and inferior frontal connectivity post-therapy. Other therapy studies support the notion that increased functional independence and segregation of resting-state networks underlies therapy success (Baliki et al. [199], Duncan and Small [200], Iorga et al. [201], Woodhead et al. [202]).
Since the application of rsfMRI is still a novel approach in aphasia therapy research, few concrete prognostic biomarkers have been identified to date. Nonetheless, given the applicability of rsfMRI with all PWA, regardless of severity, this approach presents a unique opportuni to study neurobiological predictors of therapy response. At present, it will be crucial to evaluate the predictive value of functional connectivity measures against other neural metrics (e.g., lesion data, functional activity) within a comparable modeling framework.
Structural connectivity
Aphasia is a network disorder [203,204] and middle cerebral artery strokes frequently lesion white matter fiber tracts connecting cortical language regions [205]. Prior studies have examined the relationship between structural disconnection and language function [206,207], including the AF and speech fluency [208-211]; the SLF and naming [212,213], and the uncinate fasciculus and naming [212] (for contrary findings210,213). Leveraging knowledge about the relationship between tract-disconnection and language function, connectome-based lesion symptom mapping has proven comparable to conventional lesion-symptom mapping in predicting aphasia symptoms [117,203,214-218] and to characterize spontaneous language recovery [207,219].
Encouringly, SLT has been shown to induce structural changes in the brain [177,220-222]. Specifically, therapeutic effects have been demonstrated in the RH AF in response to melodic intonation therapy [220,221], and in the LH AF [177] and inferior longitudinal fasciculus [222] in response to anomia therapy. However, baseline structural connectivity metrics have rarely been applied to predict response to therapy [118]. Bonilha et al. [118] found that greater global integrity and preserved local integration of the LH temporal lobe were strongly associated with positive language outcomes in 24 participants who underwent 30 hours of anomia therapy. Despite the scarcity of studies that have aimed to predict treated recovery from pre-therapy structural connectivity data, the findings of Bonilha et al. [118], in addition to positive findings in cross-sectional studies and studies of spontaneous recovery, warrant future research into the role of structural connectivity for treated language recovery.
Toward personalized aphasia therapy
This review described in detail the literature on predictors of therapy response in chronic aphasia. We were particularly interested in exploring the topic from the perspective of who responds to therapy and to what degree. While a review of the literature revealed a strength in the number of studies conducted within the past several decades, the literature base is severely limited in terms of the extent to which reported findings can be used to guide therapy planning and prognostication in clinical practice. The primary reason for the paucity of clinically applicable findings reported to date likely relates to the fact that most relevant studies have included too few study participants to produce results that are generalizable to the population of PWA. The consequence is most accurately described as a Prognostication Problem―i.e., clinicians are unable to adhere to a single, standardized protocol to guide prognostication with their clients [14].
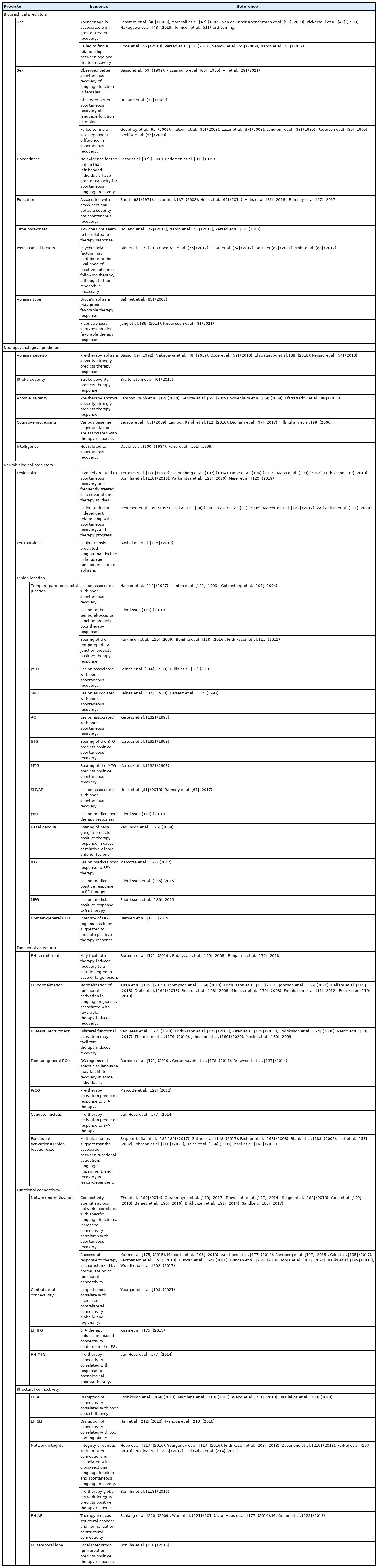
Table 1 presents an overview of the evidence reviewed above. In terms of biographical predictors, age and aphasia type were identified as potential prognostic factors, whereas the relationship between psychosocial factors and eduction, and therapy outcomes remains unclear. Sex, handedness, and time post-onset do not seem to influence treated recovery. Pre-therapy aphasia severity was the most consistently identified neuropsychological predictor, and variable measures of cognitive processing were independently associated with therapy outcomes in several studies. No evidence was found for an effect of nonverbal intelligence on language outcomes. As far as sheer volume goes, most research has been conducted on neurobiological predictors. Factors such as lesion size and location (e.g., damage to the temporoparietal junction) have frequently been associated with extent of recovery, and optimal recovery may rely on functionality of residual language regions within the LH. Similarly, integrity of the functional and structural connectomes, especially in frontal (e.g., Broca’s area) and posterior temporal areas has been found to facilitate successful response to therapy.
Collectively, although generalizable prognostic factors are scarce, these findings reveal that it is certainly possible to model therapy response based on biographical, neuropsychological, and neurobiological data collected prior to therapy. The failure of previous research to generate clinically applicable predictors does not represent that this endeavor is impossible; rather, as noted above, it is a manifestation of small sample sizes. The same issue is not as prevalent in cross-sectional studies and studies on spontaneous recovery, as these study designs typically include a greater number of participants [188,193,203] and, therefore, achieve greater statistical power. However, therapy studies are time-consuming, expensive, and require more resources. Thus, the challenge of prior studies to address the fundamental questions of who responds to therapy and to what degree likely reflects a shortage of the resources necessary to adequately address them.
Few researchers would argue against the notion that the literature clearly demonstrates the potential to improve prognostication in chronic aphasia. As a testimony to this end, the call for advancement of personalized solutions in aphasia therapy has grown immensely in recent years [15,16,105,223]. We would argue that the first step to meet the increasing demand for PAT will be to emphasize participant recruitment on a grander scale. In fact, such efforts are currently underway. Recent multisite collaborative efforts (e.g., Center for the Study of Aphasia Recovery [C-STAR], Predicting Language Outcome and Recovery After Stroke [PLORAS]) enable participant recruitment on a grander scale and, therefore, offer increased statistical power to counter the heterogeneous characteristics of this population. Further, larger sample sizes will be integral to examine how different predictors (e.g., age and lesion characteristics) support or deter recovery, and to examine how predictors interact with other therapy parameters such as dose, intensity, and therapy type. These collaborative initiatives hold tremendous promise to address some of the persistent issues described above.
The potential implications of identifying robust and generalizable predictors of therapy response in aphasia are substantial. In terms of clinical practice, improved understanding of therapy response will enable clinicians to personalize aphasia therapy more efficiently and, by extension, to enhance therapy outcomes. As for research perspectives, understanding therapy response at the individual level is fundamental for future efforts to identify what type of therapy works for whom, e.g., who is likely to benefit from pharmacotherapy, non-invasive brain stimulation, and neurofeedback training; which individuals benefit from intensive or distributed therapy; and, equally importantly, what should the focus of treatment be for individuals unlikely to respond to conventional impairment-based SLT. Thus, we believe these clear advantages of pursuing larger-scale, scientifically rigorous study designs outweigh any potential disadvantages associated with greater demand for tangible resources. Ultimately, and most importantly, these research efforts will pay dividends to PWA through substantially improved quality of life.
Conclusions
Efforts to identify robust predictors of therapy response in chronic aphasia have, as of yet, failed to generate clinically applicable findings. As a result, clinicians cannot reliably determine who is likely to respond to impairment-based therapy and to what degree, with reference to standardized clinical guidelines. Our review reveals that while several biographical, neuropsychological, and neurobiological predictors have consistently been reported, most findings cannot be generalized to the population of PWA due to use of small sample sizes. Further, inconsistent and mixed findings are a prevalent problem, likely for the same reason. Identifying reliable predictors of therapy response is a necessary prerequisite for the development of personalized therapy solutions in aphasia, i.e., therapy tailored to maximize each individual’s language recovery. Future research should aim to recruit a greater number of participants to actively facilitate the advancement of clinical management of aphasia.
Notes
Disclosure
The authors have no financial conflicts of interest.