Prediction of Early Recanalization after Intravenous Thrombolysis in Patients with Large-Vessel Occlusion
Article information
Abstract
Background and Purpose
We aimed to develop a model predicting early recanalization after intravenous tissue plasminogen activator (t-PA) treatment in large-vessel occlusion.
Methods
Using data from two different multicenter prospective cohorts, we determined the factors associated with early recanalization immediately after t-PA in stroke patients with large-vessel occlusion, and developed and validated a prediction model for early recanalization. Clot volume was semiautomatically measured on thin-section computed tomography using software, and the degree of collaterals was determined using the Tan score. Follow-up angiographic studies were performed immediately after t-PA treatment to assess early recanalization.
Results
Early recanalization, assessed 61.0±44.7 minutes after t-PA bolus, was achieved in 15.5% (15/97) in the derivation cohort and in 10.5% (8/76) in the validation cohort. Clot volume (odds ratio [OR], 0.979; 95% confidence interval [CI], 0.961 to 0.997; P=0.020) and good collaterals (OR, 6.129; 95% CI, 1.592 to 23.594; P=0.008) were significant factors associated with early recanalization. The area under the curve (AUC) of the model including clot volume was 0.819 (95% CI, 0.720 to 0.917) and 0.842 (95% CI, 0.746 to 0.938) in the derivation and validation cohorts, respectively. The AUC improved when good collaterals were added (derivation cohort: AUC, 0.876; 95% CI, 0.802 to 0.950; P=0.164; validation cohort: AUC, 0.949; 95% CI, 0.886 to 1.000; P=0.036). The integrated discrimination improvement also showed significantly improved prediction (0.097; 95% CI, 0.009 to 0.185; P=0.032).
Conclusions
The model using clot volume and collaterals predicted early recanalization after intravenous t-PA and had a high performance. This model may aid in determining the recanalization treatment strategy in stroke patients with large-vessel occlusion.
Introduction
Successful recanalization in acute stroke patients with large-vessel occlusion (LVO) is a main target of reperfusion therapy. To achieve early and complete successful recanalization, early treatment with either intravenous tissue plasminogen activator (IV t-PA) or endovascular treatment (EVT) is needed [1,2].
Even after the successful introduction of EVT, bridging therapy with IV t-PA is recommended before EVT [2]. However, IV t-PA treatment has low successful recanalization rates in patients with LVO [3-5]. In addition, bridging therapy with IV t-PA before EVT may delay the initiation of EVT or increase the risk of bleeding complications, which may lead to unfavorable outcomes [6]. In contrast, early recanalization that can be achieved with IV t-PA may be beneficial for outcomes [7]. Although bridging therapy with IV t-PA is currently a debated issue [8], a specific group of patients may benefit from IV t-PA before EVT and selection of good responders would be helpful for improving patient outcomes.
A large clot burden determined either quantitatively or qualitatively is strongly associated with successful recanalization after IV t-PA treatment [5,9-13]. In our previous study, almost all patients with thrombus measuring >200 mm3 did not achieve early recanalization after IV t-PA; these patients were suggested to be the primary candidates for direct EVT [5]. However, many patients had thrombus measuring <200 mm3, and approximately 70% of them still did not achieve early recanalization. Furthermore, predicting patients who might achieve early recanalization after IV t-PA would be helpful for deciding IV t-PA treatment in those with LVO. This suggests the necessity of an improved model that predicts not only nonrecanalization but also successful recanalization.
While a clot burden is associated with early recanalization, the collateral status may also have a role [14]. The presence of good collaterals is an indicator of a favorable outcome in patients undergoing recanalization treatment [15]. Although aiding in keeping the penumbra area durable until achieving recanalization is the main reason, an enhanced thrombolytic effect by delivering more t-PA to the clot via backflow may also contribute to the favorable outcome [14,16]. Therefore, we aimed to determine the predictors of early recanalization after IV t-PA treatment, including both clot volume and the degree of collaterals. We also aimed to develop and validate a simple prediction model for early recanalization using data from two different prospective cohorts.
Methods
Study population
We used data from two different multicenter stroke registries for developing a prediction model. For the derivation cohort, we used data from the Thrombus Imaging Study registry, which was a multicenter prospective study designed to provide clinical evidence for thrombus imaging for outcome prediction in reperfusion therapy [5]. The inclusion criteria were treatment with IV t-PA within 4.5 hours of symptom onset and visible thrombi at the occlusion site of the distal internal carotid artery (ICA) and/or middle cerebral artery (MCA) on thin-section noncontrast computed tomography (NCCT). Patients were consecutively enrolled between February 2016 and August 2017 from nine hospitals [5]. In this analysis, we included 97 patients with visible thrombus at the distal ICA or MCA (Figure 1).

Patient selection: (A) derivation cohort, (B) validation cohort. MCA, middle cerebral artery; ICA, internal carotid artery; IV t-PA, intravenous tissue plasminogen activator; SECRET, Selection Criteria in Endovascular Thrombectomy and Thrombolytic Therapy; CT, computed tomography.
For the validation cohort, we used data from the Selection Criteria in Endovascular Thrombectomy and Thrombolytic Therapy (SECRET) registry (Clinicaltrials.gov: NCT02964052) [17]. The SECRET registry is a nationwide multicenter registry for exploring the selection criteria for patients who would benefit from reperfusion therapies. This study included 1,026 patients who had been registered retrospectively from 15 hospitals between January 2012 and December 2015 and 333 patients who had been registered prospectively from 13 hospitals between November 2016 and December 2017. There were 98 patients with visible thrombi in the distal ICA and/or MCA on initial thin-section NCCT and those with imaging data available before and after IV t-PA. However, there was an overlap of the study period between the Thrombus Imaging Study and the SECRET study. Therefore, we excluded 22 patients (anonymized) who were enrolled during the overlapped period. Finally, 76 patients were included for the validation (Figure 1). This study was approved by the Institutional Review Board of each study hospital, and written informed consent was obtained from all patients enrolled prospectively.
Imaging protocol and reperfusion therapy
The patients included in this study underwent thin-section (1 or 1.25 mm) NCCT and computed tomography angiography (CTA) before IV t-PA. All patients were treated with IV t-PA (Actilyse, Boehringer-Ingelheim, Ingelheim, Germany) at a standard dose of 0.9 mg/kg (10% as a bolus followed by 90% as an infusion for 60 minutes) within 4.5 hours of stroke onset. The inclusion and exclusion criteria for IV t-PA treatment were based on the guidelines, which were not different among the study hospitals. Further EVT was sequentially performed if the patients did not show clinical improvement or did not achieve arterial recanalization after IV t-PA treatment. Follow-up CTA or magnetic resonance angiography (MRA) was immediately performed after the end of IV t-PA if the patients were not treated with EVT.
Assessment of imaging findings
The volume and density of thrombus were semiautomatically measured on baseline thin-section NCCT using three-dimensional software (Xelis, Infinitt, Seoul, Korea). The detailed methods have been previously described [5,18,19]. Briefly, when a hyperdense artery (pixels between 50 and 100 Hounsfield units) was identified on thin-section NCCT, this thrombus area was automatically shown as a red area. When clicking any portion within the thrombus area and subsequently the “Dilate” icon, every slice of thrombus was merged with automatic pixel dilation and region growing. Thereafter, the volume and density of the thrombus were shown on a screen within 1 minute by automatic calculation. Simultaneously, CTA maximum-intensity projection images and thin-section NCCT images could be synchronized on a screen, which could enable the easy identification of thrombus.
The presence of early recanalization was assessed on CTA or MRA images taken immediately at the end of IV t-PA or on digital subtraction angiography images taken during EVT. Successful recanalization was defined as a modified Thrombolysis in Cerebral Infarction (TICI) grade of 2b or 3 on digital subtraction angiography [20] or an arterial occlusive lesion score of 3 on CTA or MRA. We also determined the degree of collaterals using the Tan collateral score [11,16] based on the single-phase CTA axial image performed before IV t-PA treatment, and the presence of good collaterals was defined as a Tan score of 3. We also collected the Alberta Stroke Program Early CT score. Two stroke neurologists (Y.D.K. and J.Y.) measured the thrombus volume and density and determined the Tan score, Alberta Stroke Program Early CT score, and TICI grade. Entire imaging analyses were independently performed without knowledge of any clinical information. In the case of discrepancies, a consensus was reached for the derivation cohort and adjudicated by the imaging adjudication committee for the validation cohort.
Clinical variables
We collected data on demographics, risk factors, and premorbid disability (dependent status defined as a modified Rankin Scale score of 3–5). Time parameters such as the intervals from stroke onset to initial brain imaging, from onset to IV t-PA, and from the initiation of IV t-PA to follow-up angiographic studies were determined. Data on the total dose of t-PA for each patient were collected. The stroke severity was assessed using the National Institutes of Health Stroke Scale.
Statistical analyses
Data are presented as frequency (percentage) for categorical variables and mean±standard deviation or median (interquartile range) for continuous variables. Differences between the groups were assessed using Student’s t-test or the Mann-Whitney U-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables, as appropriate.
Univariable and multivariable logistic regression analyses were performed to determine the independent factors for early successful recanalization after IV t-PA. The collinearity of combinations of variables in the derivation cohort was evaluated using variation inflation factors (<2 being considered nonsignificant). Odds ratios (ORs) with 95% confidence intervals (CIs) for each variable included in the model were finally calculated. On the basis of the results of multivariable logistic regression analysis, we developed a prediction model and a nomogram.
We compared the predictive ability between the model including clot volume only and the model in which the presence of good collaterals was added to clot volume by computing the area under the curve (AUC) using the Delong method. The integrated discrimination improvement (IDI) index was computed to investigate whether adding the presence of good collaterals to clot volume would improve the predictive power. We also plotted decision curves to assess the net benefit of nomogram- assisted decisions.
We examined the calibration (calibration plots and Hosmer- Lemeshow test) and discrimination (AUC). To assess the external validity of model performance, we applied our prediction model to the validation cohort. We examined the performance of the final model both in the derivation and external validation cohorts in terms of discrimination using AUC and in terms of calibration by plotting the agreement between the predicted and observed probabilities across the quartiles of scores. In addition, we determined the optimal cutoff value of the prediction value based on nomogram, which was verified by calculating the Youden index.
Statistical analyses were performed using R software package version 3.6.2 (http://www.R-project.org). All statistical tests were two-tailed; P<0.05 was considered statistically significant, whereas 0.05≤P<0.2 was considered a trend toward significance to increase the sensitivity for detecting potential selection bias.
Results
Baseline characteristics
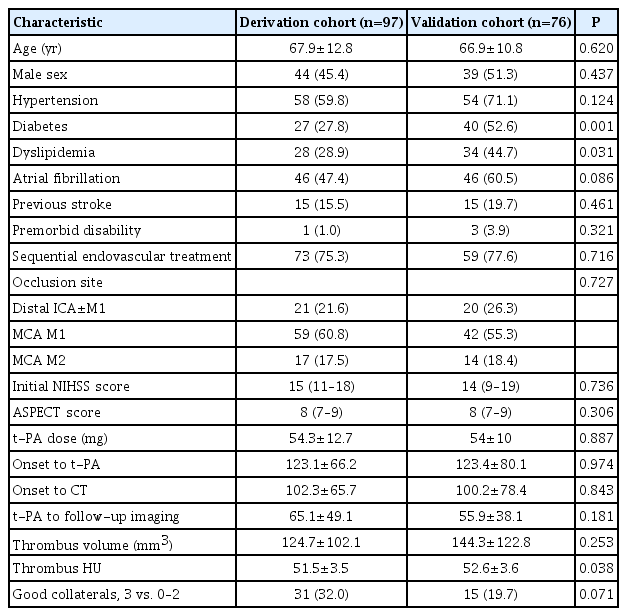
Follow-up angiographic studies were performed at a mean of 61.0±44.7 minutes after the bolus injection of t-PA (65.1±49.1 minutes in the derivation cohort and 55.9±38.1 minutes in the validation cohort, P=0.181). The baseline characteristics including clot volume and the frequency of good collaterals were not different between the derivation and validation cohorts, except diabetes and dyslipidemia, which had lower frequencies in the derivation cohort (Table 1).
Determinants for successful recanalization
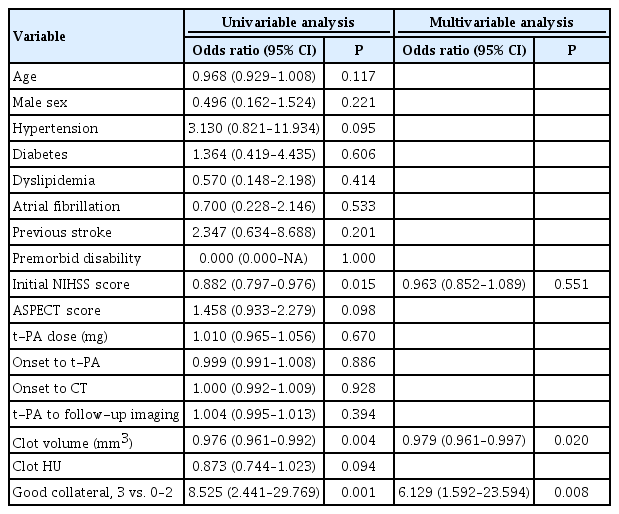
The occluded artery was recanalized immediately after IV t-PA in 15.5% (15/97) of the derivation cohort and in 10.5% (8/76) of the validation cohort. The factors associated with early recanalization in the derivation cohort were smaller clot volumes, good collaterals, and lower National Institutes of Health Stroke Scale scores on univariable analysis. Multivariable logistic regression analysis showed that clot volume (OR, 0.979 per 1 mm3; 95% CI, 0.961 to 0.997; P=0.02) and good collaterals (OR, 6.129; 95% CI, 1.592 to 23.594; P=0.008) were independent and significant predictors of early recanalization (Table 2). These findings were still observed when we divided the study population into two groups based on the timing (≥45 or ≤75 minutes) of follow-up angiographic studies after IV t-PA (Supplementary Table 1).
As the clot volume was a strong factor associated with early recanalization, we determined whether adding good collaterals to the prediction model would improve the model performance. When the presence of good collaterals was added to clot volume, the AUC improved from 0.819 (95% CI, 0.720 to 0.917) to 0.876 (95% CI, 0.802 to 0.950) in the derivation cohort (P=0.164) (Figure 2A) and from 0.842 (95% CI, 0.746 to 0.938) to 0.949 (95% CI, 0.886 to 1.000) in the validation cohort (P=0.036). The decision curves for the probability of successful recanalization in the derivation cohort showed better performance when the presence of good collaterals was added to clot volume (Figure 2B). The computation of IDI also showed significantly improved prediction (IDI, 0.097; 95% CI, 0.009 to 0.185; P=0.032). The Hosmer-Lemeshow tests were not significant in the derivation cohort (P=0.970) and the validation cohort (P=0.935), which means a good fit. Figure 3 demonstrates the predicted versus observed probability based on the quartile of the scores, which showed good correlation.

Comparison of receiver operating characteristic (ROC) curves between two models (A) and decision curve analysis (B). (A) ROC curves of the derivation cohorts and area under the curve (AUC) values (model using only clot volume, 0.819; model using both clot volume and good collaterals, 0.876). (B) Decision curve analysis showing that the model using both clot volume and good collaterals was the preferred model.
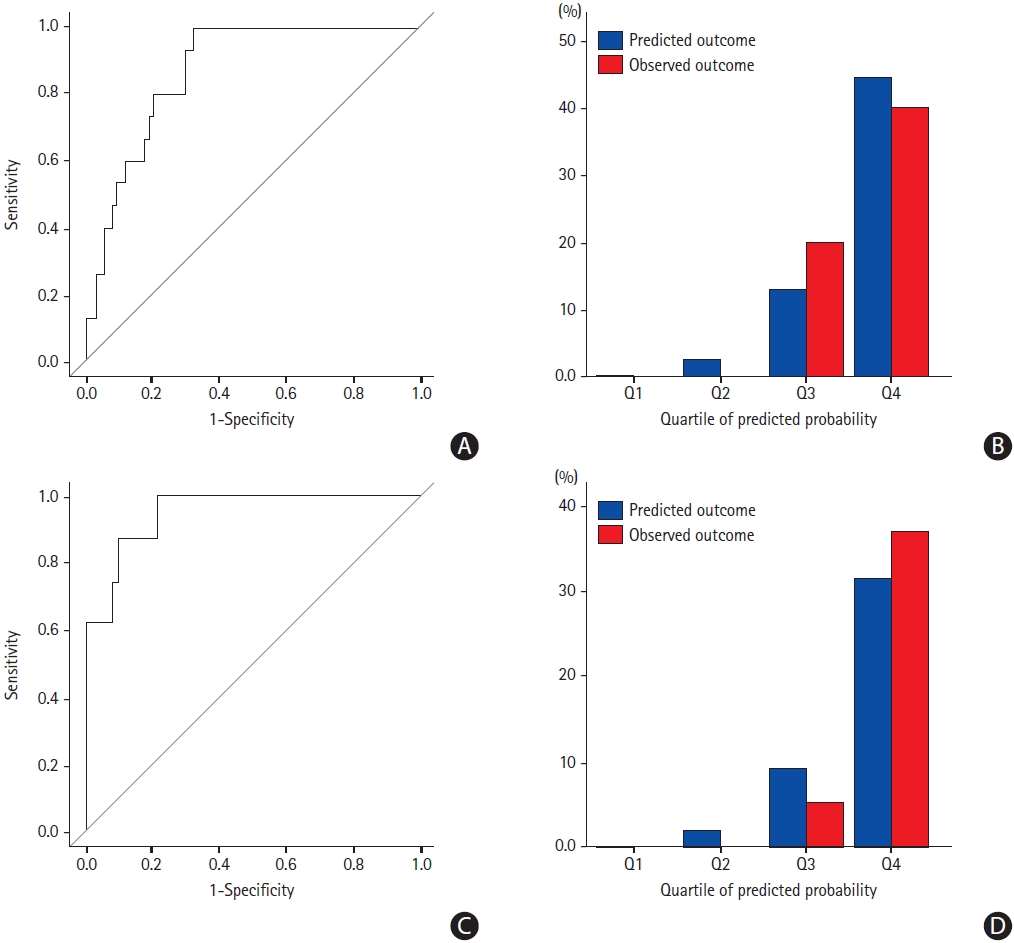
Assessment of discrimination and calibration in the derivation cohort (A, B) and the validation cohort (C, D). (A) Receiver operating characteristic (ROC) curves of the derivation cohort and area under the curve (AUC) values (AUC, 0.876; 95% confidence interval [CI], 0.802 to 0.950). (B) Calibration plot per quartile of the scores in the derivation cohort. (C) ROC curves of the validation cohort and AUC values (AUC, 0.949; 95% CI, 0.886 to 1.000). (D) Calibration plot per quartile of the scores in the validation cohort.
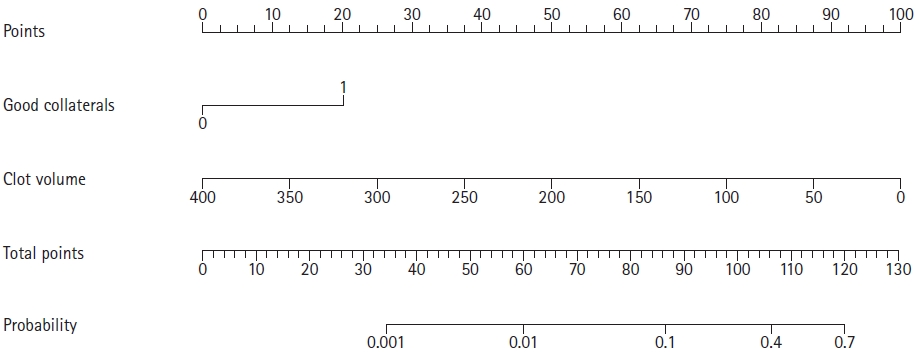
Accordingly, we developed a model predicting early recanalization after IV t-PA treatment using both clot volume and good collaterals. For easy use of the prediction model, we also developed a nomogram predicting early recanalization by assigning a graphic score to each of clot volume and good collaterals, with a point range from 0 to 20. The scores were summed to generate a total score, which was finally converted into an individual probability of early successful recanalization after IV t-PA (Figure 4). Higher total points based on the sum of the assigned number of points for good collaterals and clot volume were associated with the chance of early recanalization (Supplementary Figure 1). The cut off value of >0.1107 of the prediction model value for predicting early recanalization, which was based on the Youden index, had good sensitivity (100%) and specificity (68.3%).
Discussion
In the present study, early recanalization after IV t-PA was significantly associated with clot volume and collateral status (thus, the model was named “2C”). The prediction model using both clot volume and good collaterals could reliably predict early recanalization after IV t-PA treatment among patients with LVO. This 2C model was externally validated using another population enrolled in a nationwide multicenter registry. We also developed a nomogram using these two variables for rapid and easy use in clinical practice, which can help identify patients who would be expected to achieve early recanalization after IV t-PA treatment.
With EVT becoming the standard treatment, bridging therapy with IV t-PA before EVT in patients with LVO is a debated issue [8]. This is because, in patients with LVO, recanalization is achieved immediately after t-PA in only about 10% to 15% [4,5,13,14,19,21] and t-PA may increase the risk of bleeding and distal embolization of partially lysed clots. It may also delay the begin.
Recently, the Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients with Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals: a Multicenter Randomized Clinical Trial (DIRECT-MT) demonstrated that EVT alone was noninferior to combined IV t-PA and EVT with respect to functional outcomes, but it was associated with lower rates of successful recanalization than the combined therapy [22]. Although similar trials (NCT03192332, NCT03494920, and ISRCTN80619088) are ongoing [23,24], it is questionable whether one strategy (either direct EVT or combined intravenous thrombolysis and EVT) should be the only treatment option for all patients with LVO. A certain group of patients would benefit from IV t-PA before EVT, whereas direct EVT would be better in another group of patients [8]. In this context, prediction of early recanalization after t-PA would be helpful for deciding the strategy between direct EVT and combined intravenous thrombolysis and EVT. For example, IV t-PA before EVT may be considered when the probability of early recanalization by IV t-PA is high based on the prediction model. When the probability is very low, direct EVT may be considered as IV t-PA may increase the risk of bleeding and lead to delay the initiation of EVT.
In t-PA treatment, clot volume is an important factor associated with early recanalization. This study showed that good collaterals also enhance the chance for early successful recanalization. In a previous study based on perfusion magnetic resonance imaging, good collaterals were associated with recanalization within 3 hours after t-PA treatment [14]. Good collaterals can lead to a bidirectional action of t-PA (forward and back flow). This may be able to enhance the proteolytic action of t-PA by increasing the surface area contacting the t-PA [14,16,25].
Although there have been several prognostic models or scoring systems for estimating safety or efficacy of thrombolytic therapy with IV t-PA for acute ischemic stroke, little is known on the model for predicting the successful recanalization by IV t-PA within 1 hour. In this study, recanalization was assessed about 60 minutes after IV t-PA injection, immediately after the end of IV t-PA infusion when making a decision regarding further EVT is necessary. Therefore, prediction of recanalization at this time point is important for determining the treatment strategy. We found that only two variables (clot volume and good collaterals) were sufficient for reliably predicting recanalization with high performance at this time point. The nomogram that we developed may also be helpful for making a rapid decision.
This study had some limitations. Although collaterals are easily determined on CTA, measurement of clot volume requires a software program. Therefore, the wide use of this prediction model is limited because it depends on the availability of the software. We only included patients with occlusion of the MCA or distal ICA. Thereby, the predictive ability of our model for occlusion at other cerebral arterial segments is unknown. In addition, although we tried to show the 2C model in this study could be useful for predicting the early recanalization, the rate of early recanalization was low. This might lead the relatively low statistical power. So, we thought further analysis including larger amount of data would be necessary.
Conclusions
We showed that clot volume and good collaterals were associated with early recanalization after IV t-PA treatment, and the prediction model using clot volume and good collaterals showed high performance in the derivation and validation cohorts. Our model may aid in the decision making with respect to the recanalization strategy.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.03622.
Multivariable analyses for early recanalization and the area under curves of models by the timing of follow-up angiographic studies
Nomogram for predicting early successful recanalization after intravenous tissue plasminogen activator. When using nomogram, higher total points based on the sum of the assigned number of points for good collaterals and clot volume were associated with the chance of early recanalization. Firstly, points based on good collateral and clot volume should be calculated. Then, the summation of these points indicates the probability of the early recanalization. For example, a patient with good collaterals and clot volume of 50 mm3 would have a total of 108 points (20 points for good collaterals [red solid line] and 88 points for clot volume [red dashed line]). The predicted early successful recanalization (blue solid line) is 44.4% for this patient.
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A2A3074996); a faculty research grant of Yonsei University College of Medicine (6-2020-0202); and the “Dongwha” Faculty Research Assistance Program of Yonsei University College of Medicine (6-2019-0191).