Safety and Angiographic Efficacy of Intra-Arterial Fibrinolytics as Adjunct to Mechanical Thrombectomy: Results from the INFINITY Registry
Article information
Abstract
Background and Purpose
Data on safety and efficacy of intra-arterial (IA) fibrinolytics as adjunct to mechanical thrombectomy (MT) are sparse.
Methods
INtra-arterial FIbriNolytics In ThrombectomY (INFINITY) is a retrospective multi-center observational registry of consecutive patients with anterior circulation large-vessel occlusion ischemic stroke treated with MT and adjunctive administration of IA fibrinolytics (alteplase [tissue plasminogen activator, tPA] or urokinase [UK]) at 10 European centers. Primary outcome was the occurrence of symptomatic intracranial hemorrhage (sICH) according to the European Cooperative Acute Stroke Study II definition. Secondary outcomes were mortality and modified Rankin Scale (mRS) scores at 3 months.
Results
Of 5,612 patients screened, 311 (median age, 74 years; 44.1% female) received additional IA after or during MT (194 MT+IA tPA, 117 MT+IA UK). IA fibrinolytics were mostly administered for rescue of thrombolysis in cerebral infarction (TICI) 0-2b after MT (80.4%, 250/311). sICH occurred in 27 of 308 patients (8.8%), with an increased risk in patients with initial TICI0/1 (adjusted odds ratio [aOR], 2.3; 95% confidence interval [CI], 1.1 to 5.0 per TICI grade decrease) or in those with intracranial internal carotid artery occlusions (aOR, 3.7; 95% CI, 1.2 to 12.5). In patients with attempted rescue of TICI0-2b and available angiographic follow-up, 116 of 228 patients (50.9%) showed any angiographic reperfusion improvement after IA fibrinolytics, which was associated with mRS ≤2 (aOR, 3.1; 95% CI, 1.4 to 6.9).
Conclusions
Administration of IA fibrinolytics as adjunct to MT is performed rarely, but can improve reperfusion, which is associated with better outcomes. Despite a selection bias, an increased risk of sICH seems possible, which underlines the importance of careful patient selection.
Introduction
Despite recent advances in technical efficacy, incomplete and failed reperfusion results remain a significant concern and reduce the clinical benefit of mechanical thrombectomy (MT) [1-5]. Although the rates of thrombolysis in cerebral infarction (TICI) 3 reperfusions are constantly improving, in more than half of patients treated with MT, reperfusion is incomplete or no reperfusion is established [4-6]. Potential rescue strategies consist of intracranial-stenting [7-9], mechanical removal of small distal clots [10-12] and administration of antiplatelets [13] or fibrinolytics [14-16].
The updated 2019 American Heart Association/American Stroke Association guidelines state that the use of salvage technical adjuncts, including intra-arterial (IA) thrombolysis, may be reasonable to achieve mTICI grade 2b/3 angiographic results [17]. According to a recent survey, 39% of the responders stated to use IA recombinant tissue plasminogen activator (tPA) on individual case basis during MT [18]. Observational data suggested the use of IA fibrinolytics during MT as a therapy option in selected patients, potentially improving reperfusion [14-16,19]. However, there were mixed signals regarding a potential increase in symptomatic intracranial hemorrhage (sICH) and evidence regarding its treatment effect leading to improved reperfusion is still limited [14-16].
Aim of this multi-center analysis was to report on frequency, indication, safety and efficacy of IA fibrinolytics as adjunct to mechanical thrombectomy in consecutive patients from ten European tertiary care centers.
Methods
Patients
Ten European tertiary care centers with local prospective thrombectomy databases were invited to participate in this retrospective pooled individual patient data analysis (registry name: INtra-arterial FIbriNolytics In ThrombectomY [INFINITY]). All consecutive patients treated with MT and IA administration of alteplase (referred to as tPA) or urokinase (referred to as UK) were included. Participating centers were asked to contribute all patients in whom IA fibrinolytics were administered during or after MT for an intracranial internal carotid artery (ICA), M1 or M2 occlusion. Centers were invited to report rates of MT for these occlusion sites without additional IA fibrinolytics (Table 1). Data for all patients were collected using a standardized form with predefined variables. Local investigators completed the forms systematically using data from prospectively ascertained in-hospital thrombolysis or stroke registries enhanced with additional data from patients’ records and charts. Completed forms from all centers were sent to the coordinating center in Bern, where analyses of pooled data were performed.
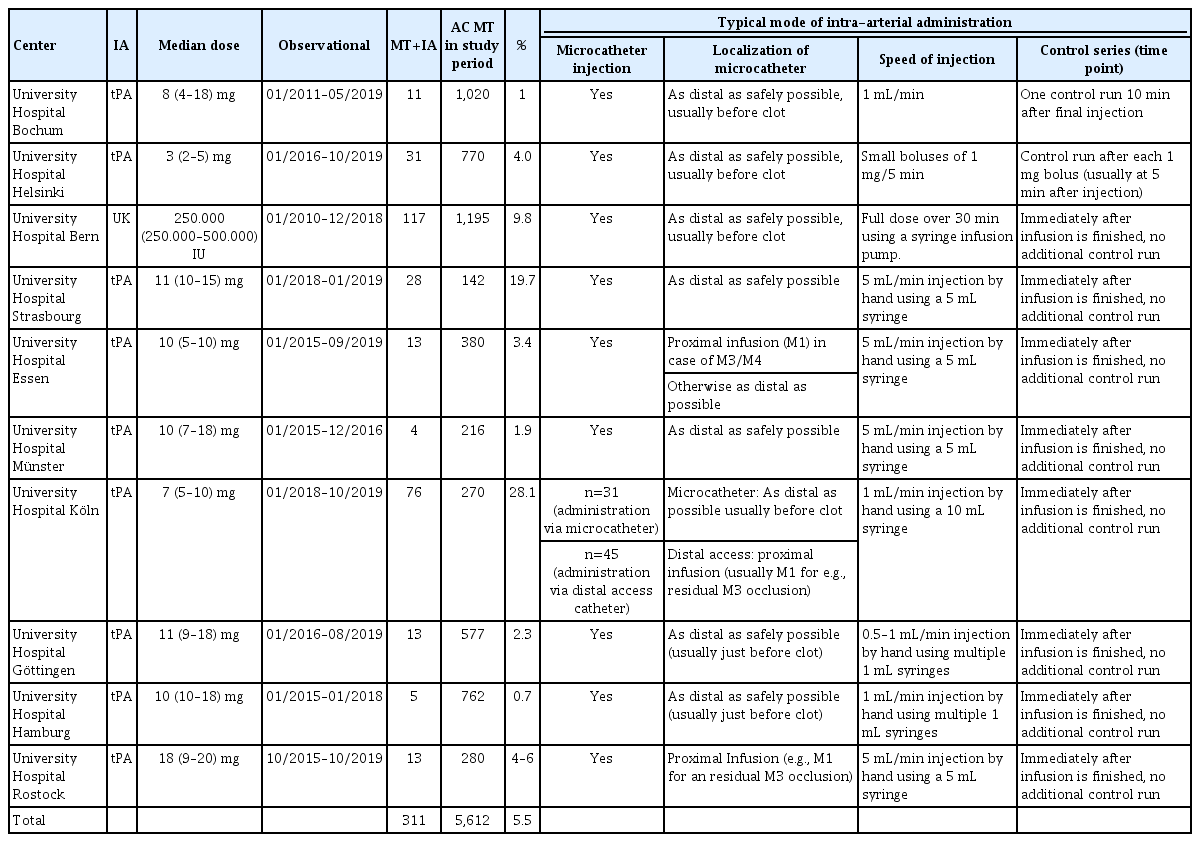
Details of participating centers, frequency of MT+IA and typical mode of intra-arterial administration
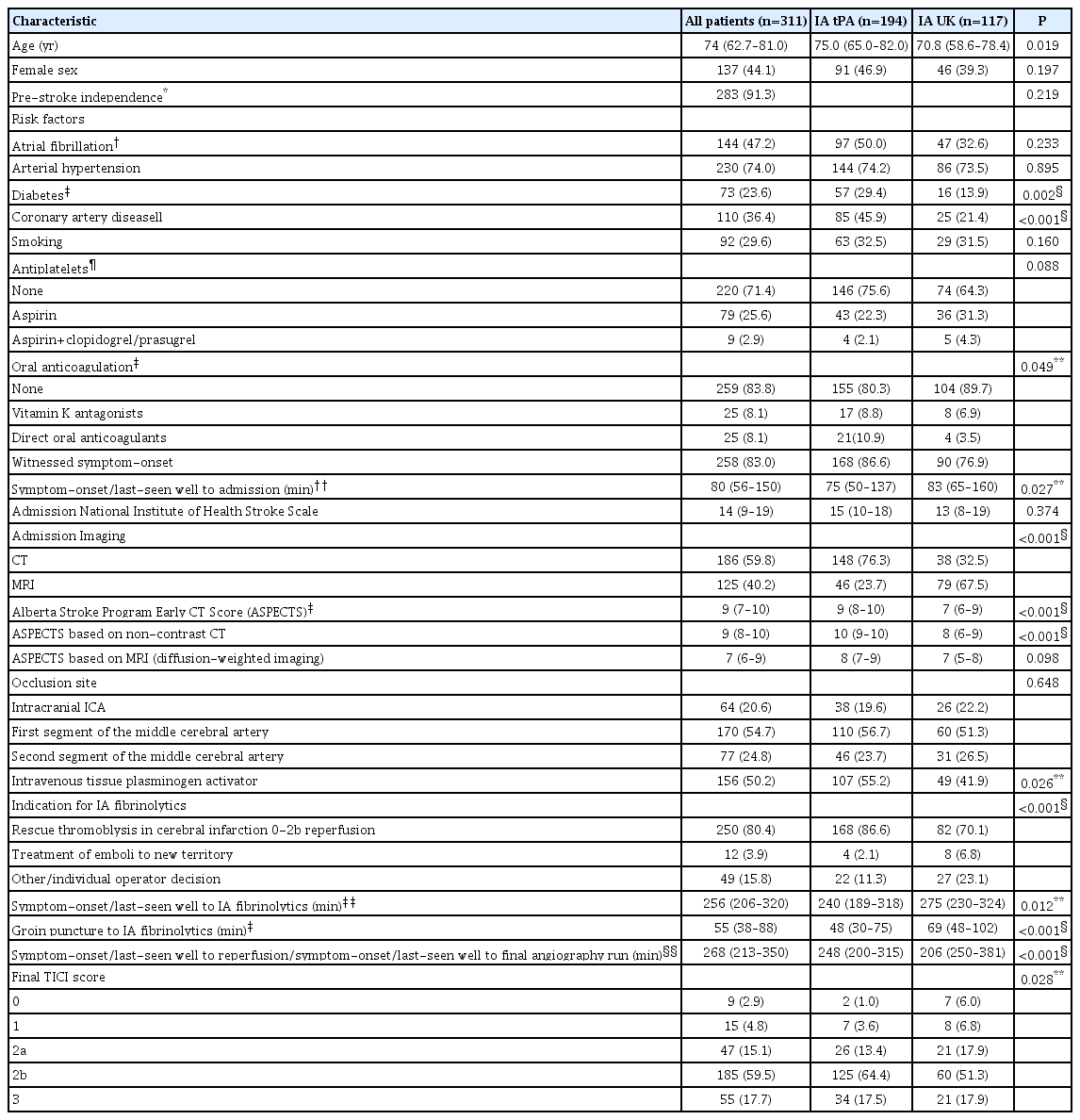
All patients were treated with approved second-generation devices, i.e., stent-retriever or large-bore aspiration catheters or a combination thereof. Ethical approval was obtained from all local ethics committees. Consent was waived according to the retrospective nature of the work, or patients gave their written or oral consent, depending on the centers ethical and institutional guidelines. Baseline characteristics and patient demographics were extracted from the centers prospective stroke databases. Functional outcome was assessed at three months using the modified Rankin Scale (mRS). The score was evaluated by a neurologist during an outpatient visit or during a structured telephone interview by a mRS-certified nurse. Functional independence was defined as mRS ≤2. Day 90 functional outcome was available for 300/311 patients (96.5%).
Image analysis
In each participating site, all images of the center’s patients treated with MT and adjunctive administration of IA fibrinolytics were reevaluated by a neurointerventionalist. Indication for administering IA fibrinolytics was determined by reviewing angiographic images and the angiographic report. For this purpose, indications were classified into (1) rescue of TICI0-2b reperfusions after MT; (2) treatment of emboli in a new territory; or (3) administration before or during the first or second stentretriever deployment at the operator’s discretion. In cases of rescue for TICI0-2b reperfusions, images were assessed before and after administration of IA fibrinolytics. For this purpose, TICI grades before and after administration of IA fibrinolytics were documented. Moreover the occurrence of any angiographic reperfusion improvement, defined as a reduction of capillary phase deficit by newly established antegrade flow, was assessed. By definition, any angiographic reperfusion improvement could therefore include cases with and without a TICI grade change. This angiographic data was available in 228/250 patients with attempted rescue of TICI0-2b, because in 22 patients no angiography runs after administration of IA fibrinolytics were performed. sICH was defined as any intracranial hemorrhage on follow-up imaging and clinical deterioration, as evidenced by an increase in the National Institutes of Health Stroke Scale (NIHSS) score of ≥4 without a mandatory confirmed causal relationship between clinical worsening and occurrence of ICH [20].
Statistical analysis
Data are presented as median (interquartile range [IQR]) or as number (%). Frequency comparisons were performed using Fisher’s exact test. Non-normally distributed continuous or ordinally scaled variables were compared using Whitney-Mann U test. For calculation of 95% confidence intervals (CIs) of frequency counts, we used the methods outlined by Clopper and Pearson [21], known as the exact binomial CI. To evaluate an association between two variables over multiple strata we calculated a Mantel-Haenszel common odds ratio (OR) and assessed heterogeneity of ORs using a Breslow-Day test. Clinical regression models were generally adjusted for age, sex, admission NIHSS, Alberta Stroke Program Early CT Score (ASPECTS), occlusion site, symptom-onset to reperfusion intervals, and TICI scores (before administration of IA fibrinolytics), according to clinical importance. For a comparison regarding IA UK versus IA tPA patients, we additionally included variables displaying significant distribution imbalances between the groups. For functional outcome, we used a binary logistic regression analysis with functional independence (mRS ≤2) as dependent variable. For an estimate regarding sICH, a binary logistic regression model was used including the same covariates. Results are displayed as adjusted OR and corresponding 95% CI. Patients with missing follow-up were excluded from the analysis regarding functional outcome or mortality. No imputation methods were performed. A sensitivity analysis of the main outcomes was performed excluding all patients treated before 2015. Statistical analyses were carried out in SPSS Statistics version 25 (IBM Co., Armonk, NY, USA) and STATA version 15.1 (StataCorp., College Station, TX, USA).
Results
Of 5,612 patients screened, 311 (median age, 74 years; 44.1% female) received additional IA after or during MT (relative frequency 5.5%; range across centers 0.7% to 28.1%) (Table 1). Patients presented with severe symptoms (median NIHSS 14; IQR, 9 to 19) and 170 patients (54.7%) were treated for an acute occlusion of the M1. Most commonly, IA fibrinolytics were administered via a microcatheter, which was positioned as distal as possible and usually just proximal to the residual occlusion site (Table 1). In 250 patients (80.4%), IA fibrinolytics were administered for rescue of a TICI0/1 in 32 patients (12.8%), for rescue of TICI2a in 54 patients (21.6%) or rescue of TICI2b in 164 patients (65.6%) reperfusions after MT. Other indications were treatment for emboli in a new territory in 12 patients (3.9%) or during first maneuvers of mechanical thrombectomy at the operator’s discretion in 49 patients (15.8%). In 117 patients (37.6%, from one center), IA UK was administered, while 194 patients (62.4%, from nine centers) were treated with IA tPA. Median dose of IA tPA was 10 mg (IQR, 5 to 10) and median dose of IA UK was 250.000 U (IQR, 250.000 to 500.000). IA tPA dose tended to be higher in patients with prior IV tPA administration (median 10 mg vs. 7.5 mg, P=0.07). For IA UK, dose regimens were higher in patients treated without prior IV tPA (median 350.000 U vs. 250.000 U, P=0.04). Of 299 patients with available data, median symptom-onset to administration of IA thrombolytics was 256 minutes (IQR, 206 to 320). Correspondingly, 127 patients (42.5%) and 43 patients (14.4%) of patients received IA fibrinolytics beyond 4.5 and 6 hours, respectively. IA fibrinolytics were administered at a median delay of 55 minutes (IQR, 38 to 88) after groin puncture. Other baseline characteristics of the cohort and stratification by IA tPA versus IA UK can be found in Table 2.
Safety evaluation
Of 308 patients with available imaging follow-up, 27 had sICH (8.8%; 95% CI, 5.9 to 12.5). Rates were numerically higher in patients treated with IA tPA as compared to patients treated with IA UK (10.3% vs. 6.1%, P=0.30). There were three missing imaging follow-ups in the UK group. Assuming a worst casescenario, the number of patients with sICH would have changed to 30 (9.6%; 95% CI, 6.6% to 13.5%). After adjustment for clinical confounders outlined in the methods section and imbalances in baseline variables between the fibrinolytics groups, the point estimate favored IA UK with a trend for fewer sICH (adjusted odds ratio [aOR], 0.53; 95% CI, 0.27 to 1.04) (Supplementary Table 1 for other regression coefficients). In the model, there was also an association of female sex (aOR, 0.30; 95% CI, 0.095 to 0.97) and M1/M2 occlusions ([aOR, 0.27; 95% CI, 0.08 to 0.86] and [aOR, 0.29; 95% CI, 0.07 to 1.14], respectively) with lower rates of sICH. Moreover, TICI grades before administration of IA fibrinolytics were associated with sICH, with a lower risk observed in better TICI grades (aOR, 0.43; 95% CI, 0.20 to 0.94 for TICI grade increase) (Figure 1). The estimates also pointed towards an increased risk of sICH in patients with pretreatment with IV tPA (aOR, 2.33; 95% CI, 0.75 to 7.17), prior use of Aspirin (aOR, 2.30; 95% CI, 0.74 to 7.09, comparator no antiplatelet medication) and those taking direct oral anticoagulants (aOR, 3.08; 95% CI, 0.55 to 17.28, comparator no oral anticoagulant medication); however, these associations did not reach statistical significance. Raw group comparisons of sICH occurrence with strata of antiplatelets and intake of oral anticoagulants can be found in Supplementary Table 2. Doses of IA tPA/UK did not differ between patients with and without sICH (median 10 mg vs. 10 mg, P=0.96; and 250,000 IU vs. 250,000 IU, P=0.48).
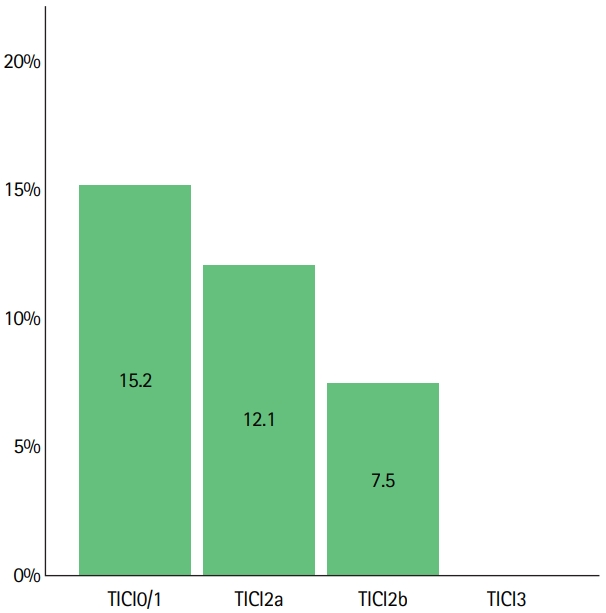
Risk of symptomatic intracranial hemorrhage (sICH) according to thrombolysis in cerebral infarction (TICI). Data on sICH was available in 308/311 patients. SICH occurred in 5/33 patients (15.2%) with TICI0/1, in 8/66 patients (12.1%) with TICI2a, in 14/187 patients (7.5%) with TICI2b and did not occur in 22 patients with TICI3. There was a decreased risk of sICH with higher TICI grade (adjusted odds ratio [aOR] per grade increase derived from logistic regression analysis: aOR, 0.43; 95% confidence interval, 0.20 to 0.94). TICI scores used were before administration of intra-arterial fibrinolytics in cases of rescue of TICI0-2b reperfusions. In other cases (treatment of emboli in new territory or administration during first retrievals at the operator’s discretion) final TICI scores were used.
After 3 months, 50 of 300 patients (16.7%) with available follow-up died, with a doubled mortality rate in patients with sICH (eight of 26 sICH patients with available follow-up, 30.8%).
Angiographic efficacy
Angiographic control runs after administration of IA fibrinolytics were available, for 228 of 250 patients (91.2%), who received IA fibrinolytics for attempted rescue of TICI0-2b reperfusions after MT. In 116 of those 228 (50.9%), any angiographic reperfusion improvement was noted, which resulted in a TICI grade improvement in 66 of 228 patients (28.9%) (Figure 2). Occurrence of any angiographic reperfusion improvement did not differ according to TICI strata before administration of IA fibrinolytics or occlusion sites (Supplementary Table 3). In 11 patients, in whom IA fibrinolytics were administered for treatment of an embolus in a new territory, successful reperfusion was reported in six patients (54.5%). In 49 patients, in whom IA fibrinolytics were administered before or during the first thrombectomy maneuvers at the operator’s discretion, final TICI grades were TICI3 in 16 patients (32.7%), TICI2b in 17 patients (34.7%), TICI2a in 13 patients (26.5%), and TICI0/1 in three patients (6.1%).

Thrombolysis in cerebral infarction (TICI) grade change after intra-arterial (IA) fibrinolytics. (A) After IA fibrinolytics, TICI shifted towards better scores (TICI grade improvement noted in 28.9% of patients). (B) Most patients with TICI2b did not improve to TICI3; however, any angiographic reperfusion improvement was relatively common in patients with initial TICI2b reperfusions (46.1%, 70/152).
Functional outcome
At 3 months follow-up, 111 of 300 patients (37.0%) achieved mRS ≤2 (Figure 3A). Functional outcomes were better in patients receiving IA UK (OR for mRS ≤2, 2.22; 95% CI, 1.37 to 3.60) (Figure 3B and C). However, this association was not statistically significant after adjustment for clinical confounders and baseline group imbalances (IA UK vs. IA tPA: adjusted OR, 1.35; 95% CI, 0.87 to 2.09). Significant variables associated with mRS ≤2 in the model were: age, admission NIHSS, TICI before administration of IA fibrinolytics, diabetes, coronary artery disease, and M2 occlusions (Supplementary Table 4 for point estimates and 95% CIs).
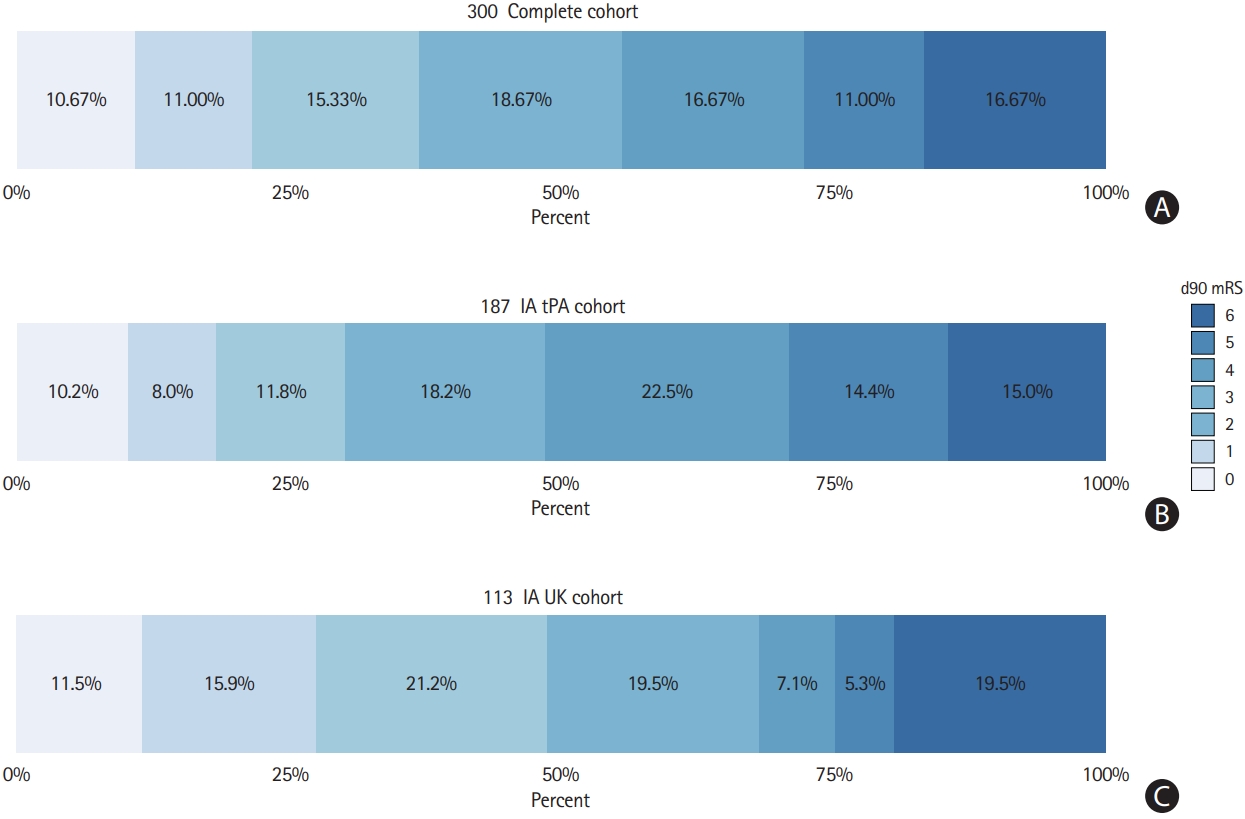
Three-month functional outcome of patients treated with mechanical thrombectomy (MT) and intra-arterial (IA) fibrinolytics. (A) Three-month functional outcome was available for 300/311 patients treated with MT+IA fibrinolytics. Functional independence (modified Rankin Scale [mRS] ≤2) was observed in 37.0% (111/300) of patients and 16.7% (50/300) of patients had died. (B, C) Functional outcomes were better in patients receiving IA urokinase (UK) (odds ratio for mRS ≤2, 2.22; 95% confidence interval [CI], 1.37 to 3.60). However, this association was not statistical significant after adjustment for clinical confounders and baseline group imbalances (IA UK vs. IA tissue plasminogen activator [tPA]: adjusted odds ratio, 1.35; 95% CI, 0.87 to 2.09).
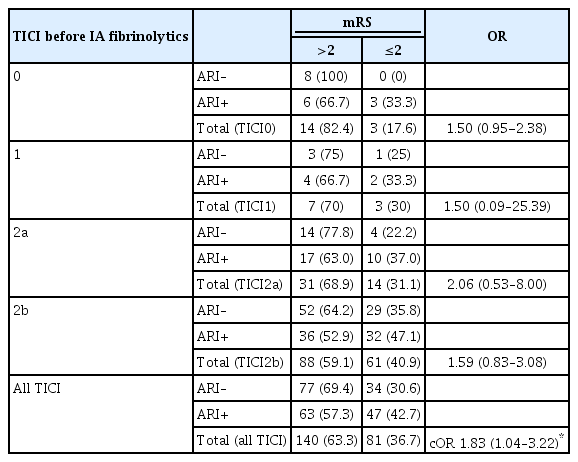
In patients with rescue of TICI0-2b reperfusions after MT and available angiographic follow-up (228 patients), any angiographic reperfusion improvement was associated with mRS ≤2 (aOR, 3.11; 95% CI, 1.41 to 6.86) (Figure 4) after adjusting for covariates outlined in the methods section. This association of functional independence and angiographic reperfusion improvement was relatively stable across strata of reperfusion grade achieved before administering IA fibrinolytics (common OR, 1.83; 95% CI, 1.04 to 3.22, P for heterogeneity of ORs, 0.54) (Table 3).
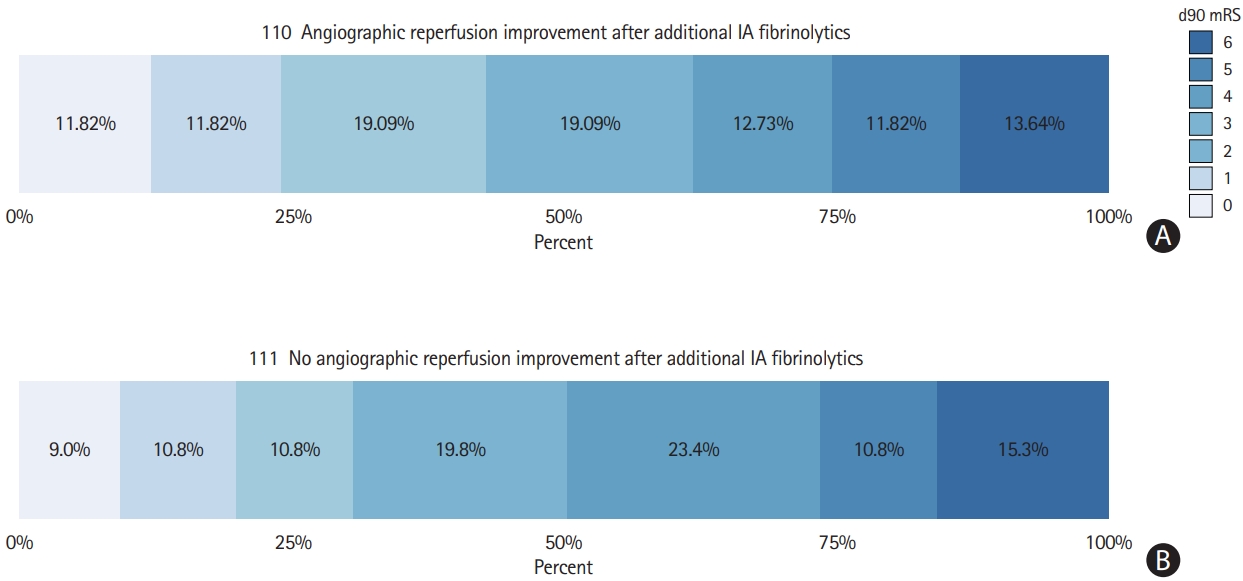
Three-month functional outcome with strata of angiographic reperfusion improvement after administering intra-arterial (IA) fibrinolytics. For 228/250 patients with an intention to improve TICI 0-2b, angiographic control runs after administration of IA fibrinolytics were available. Three-month functional outcome was available in 221 of these 228 patients. (A) Any angiographic improvement was observed in 110/221 and was associated with modified Rankin Scale (mRS) ≤2 after adjusting for covariates outlined in the methods section (adjusted odds ratio, 3.11; 95% confidence interval, 1.41 to 6.86). (B) The 111/221 patients showed no angiographic improvement. TICI, thrombolysis in cerebral infarction.
Sensitivity analysis
Excluding patients treated before 2015 lead to a subcohort of 240 patients (51 patients treated with IA UK and 189 treated with IA tPA). In this subcohort, 23 of 239 with available data had sICH (9.6%). Of 240 patients, angiographic control runs after administration of IA fibrinolytics were available for 181 of 203 patients (89.2%), who received IA fibrinolytics for attempted rescue of TICI0-2b reperfusions after MT. In these 181 patients, any angiographic reperfusion improvement was noted in 87 (48.1%). The association of angiographic reperfusion improvement with mRS ≤2 remained comparable to the complete cohort (aOR, 5.15; 95% CI, 1.91 to 13.93).
Discussion
This study has the following main findings. (1) Substantial heterogeneity regarding the frequency, dose and indications for IA fibrinolytics during or after MT can be observed across different centers. (2) In a considerable proportion of patients, IA fibrinolytics were administered after 4.5 hours, but the majority (>80%) of patients received IA fibrinolytics within 6 hours after symptom-onset. (3) Rates of sICH were twice as high compared to rates of sICH reported in the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration or the Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1) trial (8.8% vs. 3.9%/4.4%) [6,22], particularly after rescue of low TICI scores or treatment of intracranial ICA occlusions. (4) Any angiographic reperfusion improvement was observed in half of the patients with around one-third resulting in a TICI grade change. (5) Any angiographic reperfusion improvement after IA fibrinolytics was associated with better outcomes.
Frequency and indications
Depending on the survey, 39% and 60.6% of survey respondents stated using IA thrombolytics in contemporary MT practice [18,23]. In our multi-center analysis, the overall rate of IA fibrinolytics administrations was only 5.5% of all anterior circulation large-vessel occlusion MT cases, corroborating the survey’s finding that around 50% of interventionalists only treat 1 to 5 cases/year with IA rtPA [23]. However, considerable heterogeneity in IA fibrinolytic frequency was noted across centers, with some centers utilizing IA tPA in every fourth patient undergoing MT. Median dose of IA tPA was 10 mg, which is within the range of previous observational reports [15] and dosage regimens applied in the THRombectomie des Artères CErebrales (THRACE) trial (mean dose, 8.8 mg) [24]. In line with the Prolyse in Acute Cerebral Thromboembolism II (PROACT-II) inclusion criteria, most patients were treated within 6 hours after symptom-onset, but a considerable proportion of patients was treated beyond 4.5 hours [25]. Treatment outside of IV tPA eligibility criteria was also evident considering the inclusion of very low ASPECTS cases (n=19, ASPECTS <5) and treatment of patients with prior anticoagulation (16%).
Risk of bleeding
sICH has always been a major concern in patients treated with IA fibrinolytics. Meta-analysis of randomized-controlled trials (RCTs) evaluating IA fibrinolytics as stand-alone approach observed a 6.6% excess risk of sICH in patients treated with IA fibrinolytics as compared to controls (OR, 2.87; 8.9% vs. 2.3%, number needed to harm 15) [26]. However, no excess in mortality was found (20.5% vs. 24.0%) [26]. In this multi-center single-arm observational cohort, we found an overall sICH rate of 8.8% (95% CI, 5.9 to 12.5). This compares unfavorable to rates of sICH reported in HERMES (4.4%) [22] and ESCAPE-NA1 (3.9%) [6]. However, there are many constraints associated with a direct comparison. First, this observational cohort included patients with pre-stroke disability, patients under oral anticoagulation and lower ASPECTS as compared to recent RCTs. Second, and maybe most importantly, there was a severe selection bias towards poor reperfusion grades and hence, lengthy procedure, because an incomplete reperfusion status was the main indication for administering IA fibrinolytics. Patients in whom no reperfusion or only incomplete reperfusion can be achieved have significantly higher rates of sICH [27-29], and this association was also observed in our data.
Nevertheless, known excess risk from IA fibrinolytics RCTs and the possibility of an up to tripled sICH risk in this study (considering the upper 95% CI reported here) should prompt very careful patient selection. This comprises identification of patients with an a priori high risk of sICH, including patients with initial TICI0/1 and intracranial ICA occlusion as observed in our cohort. Also other, more general factors associated with sICH in other MT cohorts like history of diabetes, high blood pressure, age, high NIHSS, low ASPECTS, and poor collaterals should be acknowledged [27,29]. The lack of a statistically significant association of sICH and prior IV tPA, Aspirin or anticoagulant intake should not be mistaken for clear safety signals, as the number of patients included in these subgroups were small, uncertainty is high and the point estimates were directed towards an increased risk. We did not observe a clear association of dose-risk effects, which has been reported by others [30]. However, as dose-regimens were not randomized, we cannot exclude that doses applied were modified according to the patients presumed risk of bleeding, including pretreatment with IV tPA.
Surprisingly, the rate of sICH appeared lower after IA UK as compared to IA tPA, which seemed tangible after adjustment for clinical confounders and baseline imbalances between the groups. There is some preclinical evidence that UK upregulates blood brain barrier tight junctions and has a more favorable effect on matrix metalloproteinases, potentially lowering the risk of bleeding in comparison to tPA [31,32]. However, we want to stress that this result was not statistically significant and should be interpreted cautiously. Patients treated with IA UK were treated at one center only, while IA tPA patients were treated at nine different European centers. While we have tried to account for baseline differences between the groups, there is considerable room for hidden bias reflecting the centers individual decisions and practice to select patients for MT+IA fibrinolytic treatments.
Angiographic reperfusion improvement
Any angiographic reperfusion improvement was observed in approximately 50% of patients. These results corroborate previous observations, showing that administration of IA fibrinolytics improved reperfusion after failed MT [14]. Notably, angiographic control runs were usually performed immediately after termination of the IA fibrinolytics infusion. Despite this promising observation and plausible causality, there is some uncertainty regarding the degree of this observation caused by IA fibrinolytics. Theoretically some of the observed residual capillary perfusion deficits may also dissolve spontaneously (i.e., without additional administration of IA fibrinolytics), if angiographic control runs would have been performed with a considerable delay in patients without additional administration of IA fibrinolytics.
On the other hand, we were unable to evaluate delayed reperfusion after administration of IA fibrinolytics and hence, the observed efficacy may seem artificially low. Despite a confinement to the immediate post-infusion period, however, angiographic reperfusion improvement was associated with superior functional outcomes and a 12.1% absolute increase of mRS ≤2, which underlines the notion that even small reperfusion improvements may be clinically significant.
Outlook
Almost half of the interventionalists believe that IA thrombolysis may have a role in modern endovascular practice, but current evidence is still limited [23]. Currently, there are two ongoing trials evaluating IA fibrinolytics as adjunct to MT. The Boosting REcanalization of Thrombectomy for Ischemic Stroke by Intraarterial TNK (BRETIS-TNK) pilot study (clinicaltrials.gov, NCT04202458) will assess the safety and efficacy of IA tenecteplase continuously administered after the first attempt of a thrombectomy device pass. The CHemical OptImization of Cerebral Embolectomy (CHOICE) trial will randomize patients with TICI2b reperfusion to receive either a 30-minute IA infusion of weight-adapted tPA or IA placebo [33]. The present study suggests that although TICI grade improvement occurs only in one-third of patients, even small non-TICI grade relevant angiographic perfusion improvements are associated with favorable outcomes. Because in our single-arm cohort we could not exclude a potentially up to tripled risk of sICH, future observational studies should compare patients with MT+IA to patients with MT and failed or incomplete reperfusion without additional rescue IA fibrinolytics. Such studies may also incorporate an assessment of delayed reperfusion, which’s rates remain unknown from currently available analyses.
Limitations
This is a retrospective single-arm observational study with its associated limitations. First, angiographic efficacy analyses were performed at each center independently, giving rise to inter-rater variability. Second, generalizability of the findings is limited, because the IA+MT cohort constitutes only 5.5% of all patients treated for anterior circulation large vessel occlusion at the ten participating centers. Hence, there is a very high chance of selection bias and rates of sICH may be even higher in an unselected cohort. Third, eleven patients (3.5%) were lost to follow-up regarding functional outcomes, leading to attrition bias. Finally, comparison of rates of sICH with RCT trial data is limited by differences in baseline patient characteristics and a selection bias towards poor reperfusion grades.
Conclusions
Administration of IA fibrinolytics as adjunct to MT is performed rarely, but can improve reperfusion, which is associated with better outcomes. Even when considering a selection bias favoring patients with poor reperfusion, an excess risk of sICH seems possible, which should prompt careful patient selection, particularly in patients with intracranial ICA occlusions or attempted rescue of a TICI0/1 reperfusion. Potential differences regarding the rates of sICH between IA UK and IA tPA after MT warrant further studies.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.01788.
Logistic regression model (dependent variable symptomatic intracranial hemorrhage)
Risk of symptomatic intracranial hemorrhage with strata of prior antithrombotic and oral anticoagulant intake
Any angiographic reperfusion improvement with strata of initial reperfusion success and occlusion site
Logistic regression model (dependent variable mRS ≤2)
Notes
Disclosure
Johannes Kaesmacher reports grants by the Bangerter-RhynerFoundation and the Swiss Academy of Medical Sciences related to the project. Urs Fischer reports grants from Medtronic during the conduct of the study, grants and other from Medtronic, and other from Medtronic, Stryker, and CSL Behring outside the submitted work. Jan Gralla reports grants from Medtronic and other from Penumbra outside the submitted work. All other authors report no conflict of interests.
Acknowledgements
The research was supported by the Bangerter-Rhyner-Foundation and the Swiss Academy of Medical Sciences (Johannes Kaesmacher, grant paid to institution). The funder did not take part in conceptualization, drafting or approval of the manuscript.