Role of Blood Pressure Management in Stroke Prevention: A Systematic Review and Network Meta-Analysis of 93 Randomized Controlled Trials
Article information
Abstract
Background and Purpose
The present study aimed to compare the efficacy and tolerability of different blood pressure (BP)-lowering strategies.
Methods
Randomized controlled trials that compared various antihypertensive treatments and stroke outcomes were included. Eligible trials were categorized into three scenarios: single or combination antihypertensive agents against placebos; single or combination agents against other agents; and different BP-lowering targets. The primary efficacy outcome was the risk reduction pertaining to strokes. The tolerability outcome was the withdrawal of drugs, owing to drug-related side effects (PROSPERO registration number CRD42018118454 [20/12/2018]).
Results
The present study included 93 trials (average follow-up duration, 3.3 years). In the pairwise analysis, angiotensin-converting enzyme inhibitors (ACEis) and beta-blockers (BBs) were inferior to calcium channel blockers (CCBs) (odds ratio [OR], 1.123; 95% confidence interval [CI], 1.008 to 1.252) (OR, 1.261; 95% CI, 1.116 to 1.425) for stroke prevention, BB was inferior to angiotensin II receptor blockers (ARB) (OR, 1.361; 95% CI, 1.142 to 1.622), and diuretics were superior to ACEi (OR, 0.871; 95% CI, 0.771 to 0.984). The combination of ACEi+CCB was superior to ACEi+diuretic (OR, 0.892; 95% CI, 0.823 to 0.966). The network meta-analysis confirmed that diuretics were superior to BB (OR, 1.34; 95% CI, 1.11 to 1.58), ACEi+diuretic (OR, 1.47; 95% CI, 1.02 to 2.08), BB+CCB (OR, 2.05; 95% CI, 1.05 to 3.79), and renin inhibitors (OR, 1.87; 95% CI, 1.25 to 2.75) for stroke prevention. Regarding the tolerability profile, the pairwise analysis revealed that ACEi was inferior to CCB and less tolerable, compared to the other treatments.
Conclusions
Monotherapy using diuretics, CCB, or ARB, and their combinations could be employed as first-line treatments for stroke prevention in terms of efficacy and tolerability.
Introduction
Stroke is a leading cause of morbidity and mortality across the globe. In 2017, stroke was the second most frequent cause of death, after ischemic heart disease, and caused 6.2 million deaths worldwide [1]. Moreover, hypertension is a leading cause of stroke and the significance of blood pressure (BP) lowering in stroke prevention is already established in literature [2]. Considering the high prevalence of stroke, achievement of the most appropriate or ideal BP could have a significant impact on public health.
A recent meta-analysis reported that the reduction in systolic blood pressure (SBP) by 10 mm Hg was associated with a 27% reduction in the risk associated with stroke [3]. Moreover, the magnitude of reduction in BP was linearly associated with the extent of risk reduction pertaining to recurrent strokes [4]. A systematic review of the 2017 American College of Cardiology (ACC)/American Heart Association (AHA)/American Academy of Physician Assistants (AAPA)/Association of Black Cardiologists (ABC)/American College of Preventive Medicine (ACPM)/American Geriatrics Society (AGS)/American Pharmacists Association (APhA)/American Society of Hematology (ASH)/American Society for Preventive Cardiology (ASPC)/National Medical Association (NMA)/Preventive Cardiovascular Nurses Association (PCNA) guidelines for the prevention, detection, evaluation, and management of high BP in adults (the 2017 high BP guidelines) has recommended intensive BP-lowering treatments (to a target of below 130 mm Hg) other than the standard antihypertensive therapies [5]. However, despite the well-established and widespread use of BP-lowering agents for the prevention of stroke, the most appropriate treatments pertaining to various populations are still under debate. A meta-analysis published in 2016 demonstrated that calcium channel blockers (CCBs) were superior to other drugs for the prevention of stroke in the general population [3]. A systematic review of the 2017 high BP guidelines employed network meta-analysis and reported that thiazides and thiazide-like diuretics (THZs) were associated with a significantly lower risk of stroke in patients with hypertension [5]. Another meta-analysis reported that CCBs were at least as effective as the other first-line antihypertensive agents in the management of hypertensive patients with a previous history of stroke [6]. Nevertheless, previous meta-analyses have rarely involved the comprehensive analysis of the most appropriate antihypertensive agents for different target populations. Moreover, previous meta-analyses did not consider the tolerability and safety profiles pertaining to the antihypertensive strategies. Furthermore, combined antihypertensive strategies were recommended by several guidelines, in order to achieve better BP control and to slow the progression of hypertension. However, previous studies provided limited evidence on the efficacy of combined antihypertensive therapies in stroke prevention.
Traditional meta-analysis could only compare the treatments assessed in the same study, whereas network meta-analysis could compare multiple treatments from different studies through common comparators. Consequently, several treatments could be ranked [7,8]. Hence, the present study performed a network meta-analysis to compare the efficacy and tolerability profiles of both single and combined antihypertensive strategies for stroke prevention in different populations.
Methods
Search strategy and selection criteria
The present meta-analysis adhered to the preferred reporting items for systematic reviews and meta-analysis PRISMA) statement [9] and the PRISMA network meta-analysis extension statement [10]. The current study used an existing strategy [11] with additional items, such as cerebrovascular disorders, stroke, brain infarction, cerebral infarction, brain ischemia, cerebral hemorrhage, or intracranial hemorrhage, in order to identify the relevant trials from the Pubmed database, published during the time period from January 1, 1966 to December 1, 2018. The detailed search terms are provided in Appendix 1. The present study restricted the search to randomized controlled trials (RCTs) alone without any language restrictions. The Cochrane Collaboration database was also searched. Furthermore, in order to identify eligible studies, the present study performed a manual inspection of the reference list pertaining to the studies included in the review. Subsequently, a manual examination was performed to ascertain whether each trial reported stroke as a primary or secondary outcome. Studies that fulfilled the following criteria were included in the current meta-analysis: (1) RCTs; (2) greater than 1,000 patient-years of follow-up in each study group; (3) trials that reported stroke as the primary or secondary outcome; and (4) trials that used antihypertensive drugs for indications other than the management of hypertension such as proteinuria. Eligible trials were extracted and categorized into three scenarios: single, or a combination antihypertensive agents against placebos, single, or combination agents against others, and different BP-lowering targets. Trials that documented the presence of baseline comorbidities were not excluded.
Data extraction and quality assessment
The literature search, data extraction, and quality assessment were performed independently by two researchers (X.L.Z. and Y.D.). In case of disagreements, consensus was achieved through the referral to a third reviewer (J.T.Y.). Data were extracted into specially designed Excel sheets that listed the baseline characteristics pertaining to each group, which is provided in Supplementary Table 1.
The primary efficacy outcome was measured by the incidence of stroke. Outcomes of interest were all-cause mortality, cardiovascular-related deaths, all strokes (fatal or nonfatal), fatal or disabling stroke, ischemic stroke, and hemorrhagic stroke by groups. The tolerability outcome was measured by the withdrawal, owing to drug-related side effects.
The quality of each study was critically appraised by the two researchers who performed the literature review, on the basis of a 7-point tool, in order to assess the risk of bias using the Cochrane Collaboration tool [12].
Statistical analysis
The present study performed the meta-analysis in two steps. First, a traditional meta-analysis was performed to clarify the effects of antihypertensive agents on the odds ratio (OR) of various outcomes. Second, a pairwise and network analysis was performed to compare the efficacy and tolerability of all antihypertensive agents in stroke prevention.
Effects of BP-lowering for various outcomes
In this step, the present study combined the trials involving antihypertensive agents versus placebos and higher versus lower BP-lowering targets, and performed a traditional meta-analysis. The OR was estimated from the number of events and participants pertaining to each outcome in each trial and pooled results with the Mantel-Haenszel and Hartung-Knapp adjustment for random effects models. The magnitude of the statistical heterogeneity among the studies was assessed using the standard Cochrane chi-square test. Subgroup analyses were stratified by age, history of stroke, cardiovascular disease, diabetes mellitus, baseline SBP levels, and achieved SBP level. Publication bias was evaluated both graphically using a funnel plot and using the Egger statistical test for funnel plot asymmetry [13], if a minimum of 10 studies were available for each outcome. A leave-one-out sensitivity analysis was performed to determine whether any one study had a disproportionately large impact on the pooled OR.
Pairwise and network analysis of BP-lowering agents for stroke prevention
In this step, the current study included all the eligible trials and performed the pairwise and network meta-analysis. The primary outcome was measured as all types of stroke reduction and the tolerability outcome was assessed by the incidence of drug withdrawal, owing to drug-related side effects. First, a pairwise meta-analysis was performed with a random effects model to analyze direct treatment comparisons. Heterogeneity was assessed using the I2 metric. Second, the present study analyzed the pooled data pertaining to all BP-lowering treatments with random effects models within a Bayesian framework in OpenBUGS (http://openbugs.net) [14]. The details pertaining to the OpenBUGS codes that were used in the study are shown in Appendix 2. A valid network meta-analysis will satisfy the assumption of transitivity. Differences between the direct and indirect comparisons could suggest that the transitivity assumption might not hold. The present study assessed the evidence consistency in the networks in two ways. One was the nodesplit approach to contrast direct evidence with indirect evidence from the entire network on each node [15-17]. The other was the design-by-treatment interaction model that provided a single inference, using the chi-square test, regarding the plausibility of assuming consistency throughout the entire network [18]. The surface under the cumulative ranking curve (SUCRA) and rankograms were used to provide a hierarchy of the regimens [19]. The two-dimensional plots and clustering methods were conducted to obtain meaningful groups of the treatments [20]. In addition, the current study assessed the small study effects using comparison adjusted funnel plot symmetry [20].
Sensitivity analyses
In order to examine the generalizability of the findings, the present study assessed for the effects of different trials and participant characteristics on the outcomes of sensitivity analyses by restricting the analyses to studies with the following design characteristics: hypertensive participants, no heart failure, published in or after 2000, and duration of follow-up of more than 3 years. The present study performed the subgroup analyses, in accordance with the age, history of stroke, history of diabetes, and baseline SBP. More details about the statistical analysis are shown in Supplementary methods.
Traditional meta-analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) and network meta-analyses were performed using OpenBUGS 3.2.3 and STATA 14.0 (StataCorp., College Station, TX, USA).
Results
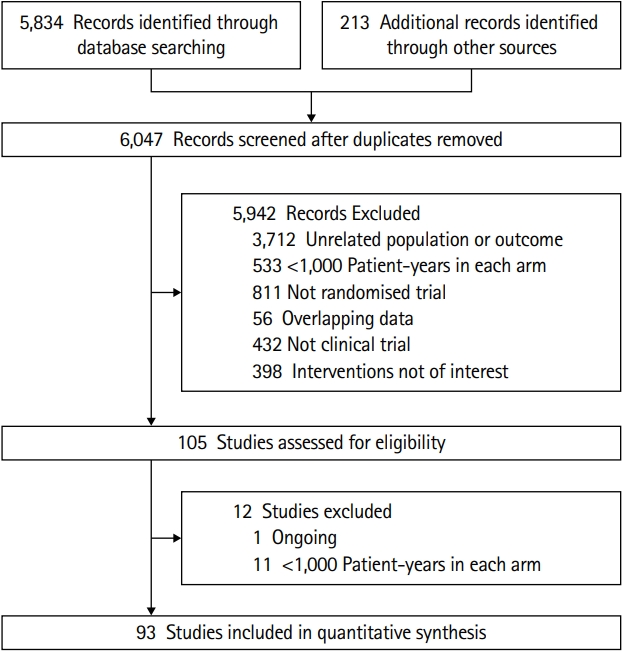
In the present study, a total of 93 RCTs met the inclusion criteria, which enrolled 504,613 participants with an average follow-up period of 3.3 years and provided sufficient data to be included in the traditional or network meta-analysis (Figure 1). Among the aforementioned studies, 66 were deemed to be trials pertaining to the lowering of BP (52 compared a single BP-lowering agent against a placebo; 14 compared different BP-lowering targets) and they were included in the analysis to explore the association between the BP-lowering treatments and various outcomes. Among the 64 studies, 44 focused on patients above the age of 60 years. Four studies involved participants without any prior history of stroke and five studies included participants with a previous history of stroke. A total of 82 trials with 14 different BP-lowering strategies were included to compare the efficacy of the treatments. Six drug classes, alone or in combination, were compared with each other or the placebos. Among the 82 studies, five studies focused on participants without any prior history of stroke, whereas seven studies included patients with a previous history of stroke. Among the studies, 60 trials were published after 2000 and 42 studies focused on participants with hypertension. The present study included 22 trials that reported the events pertaining to drug-related side effects and withdrawal, in order to compare the tolerability of the treatments. In the current study, 36 trials compared different BP-lowering agents against each other and nine of them were included in both the analyses. Among them, five trials compared the BP-lowering agents to placebos and four trials compared the different BP-lowering targets with different antihypertensive agents.
Regarding the quality of the studies, 88 trials were judged to be at a low risk of bias; the risk of bias was unclear in three trials and two trials were deemed to be at a high risk of bias. The baseline characteristics and summary of risk bias assessment of the trials are shown in Supplementary Table 1 and Supplementary Figure 1.
Meta-analysis of the association between BP-lowering treatment and various outcomes
The significance of SBP reduction pertaining to various outcomes is shown in Figure 2. BP-lowering treatment was associated with a significant risk reduction in all strokes (OR, 0.79; 95% confidence interval [CI], 0.74 to 0.85). Consistently, the reduction in BP was associated with a reduction in all-cause mortality, cardiovascular-related death, fatal or disabling stroke, ischemic stroke, and hemorrhagic stroke. The Q statistics and I2 metrics indicated that the heterogeneity pertaining to all the concerned outcomes was moderate (Supplementary Figures 2-7).
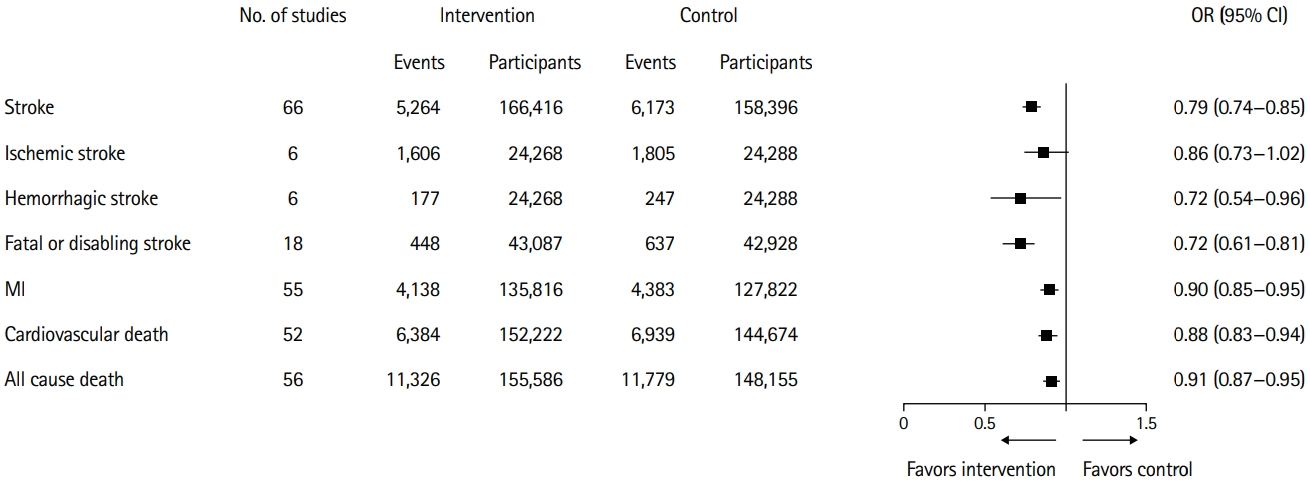
Significance of systolic blood pressure reduction pertaining to multiple outcomes. OR, odds ratio; CI, confidence interval; MI, myocardial infarction.
The association between BP reduction and stroke prevention, categorized according to the different study characteristics, is shown in Figure 3. The results of the subgroup analyses were generally concurrent with the main analyses, which showed a significant association between the stroke incidence and BP-lowering treatments. However, among the patients with baseline SBP below 130 mm Hg, there was no significant association between the stroke incidence and BP-lowering treatments (OR, 1.07; 95% CI, 0.84 to 1.36). The present study observed a trend of increased benefits that could be gained with the increase in baseline SBP. Moreover, BP-lowering treatments were associated with a low risk of stroke at all target levels of SBP, except for the levels of 120 to 129 mm Hg. The present analyses showed a potential benefit pertaining to the association between BP reduction and stroke prevention in patients without a previous history of stroke. However, the results were not statistically significant (OR, 0.80; 95% CI, 0.53 to 1.21). Heterogeneity pertaining to the subgroups, measured using I2, is demonstrated in the subgroup plots in Supplementary Figures 8-13.
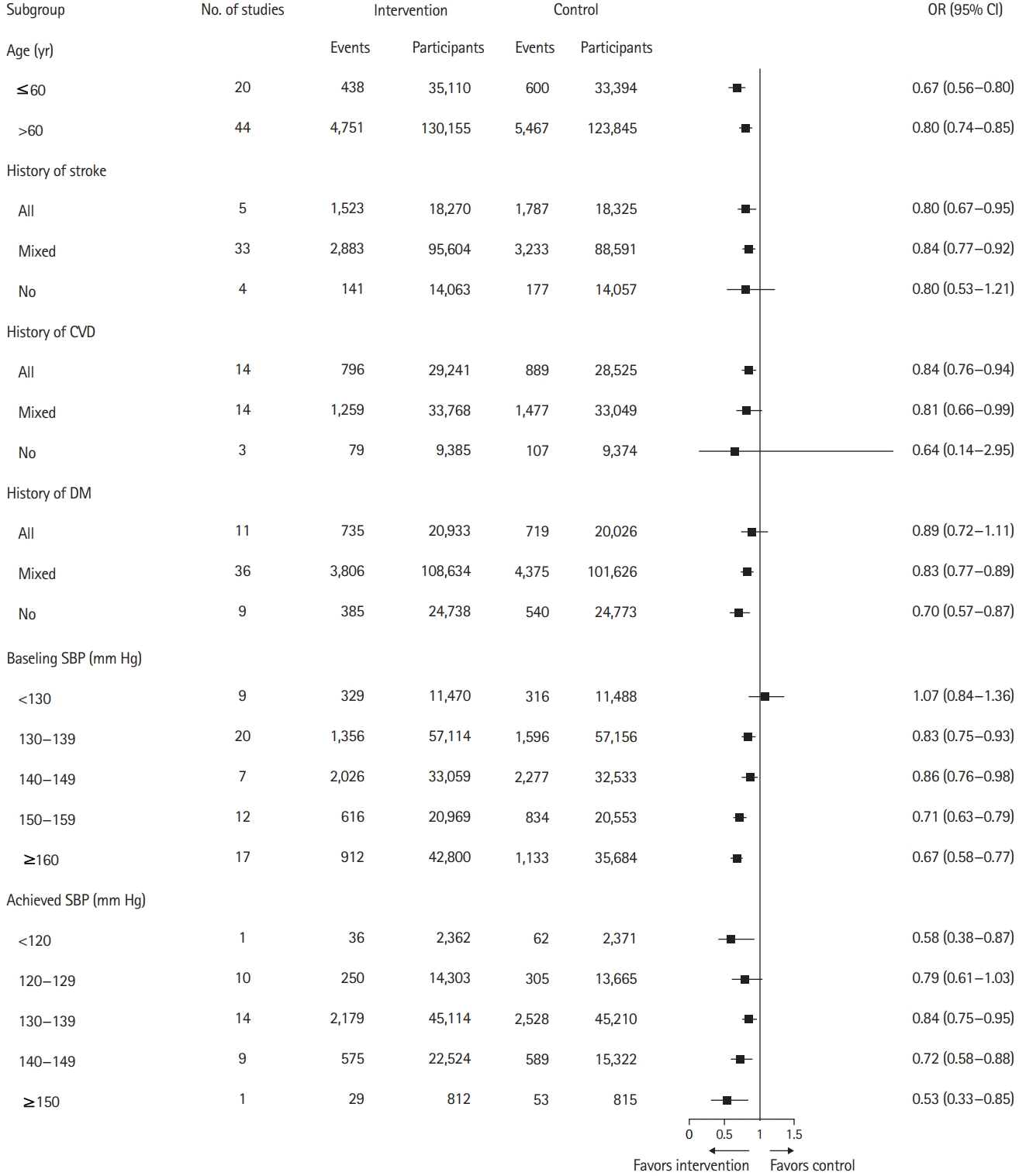
Association of blood pressure lowering and stroke prevention, categorized in accordance with the multiple study characteristics. OR, odds ratio; CI, confidence interval; CVD, cardiovascular disease; DM, diabetes mellitus; SBP, systolic blood pressure.
The possibility of publication bias was analyzed using Funnel-plot-based methods, which showed statistical significance pertaining to the outcomes of stroke and all-cause mortality (Egger’s test, P=0.03 and P=0.02, respectively). The Duval and Tweedie trim and fill procedure suggested little changes in the OR and 95% CI after the adjustment (OR, 0.82; 95% CI, 0.80 to 0.88) for stroke and (OR, 0.94; 95% CI, 0.90 to 0.97) all-cause mortality (Supplementary Figures 14-21).
A leave-one-out sensitivity analysis was performed and the pooled OR slightly varied from the original analysis (ranging from 0.91 to 0.92 for all-cause mortality; 0.88 to 0.89 for cardiovascular-related death; 0.79 to 0.80 for all stroke subtypes; 0.69 to 0.72 for fatal or disabling stroke; 0.83 to 0.89 for ischemic stroke; and 0.67 to 0.81 for hemorrhagic stroke) (Supplementary Figures 22-27). Hence, the effect of any one study on the overall summary estimates remained low.
Comparison of different BP-lowering treatments using pairwise and network meta-analysis
A total of 82 studies were included in the BP-lowering treatment comparison. Pairwise and network meta-analyses were performed to analyze the efficacy and tolerability as outcomes. Networks of eligible comparisons for efficacy and tolerability are presented in Figure 4, showing predominantly pairwise comparisons of agents with CCB, angiotensin II receptor blocker (ARB), angiotensin-converting enzyme inhibitor (ACEi), or placebo. Thirty pairwise treatment comparisons had direct evidence pertaining to efficacy and 11 pairwise treatment comparisons had direct evidence pertaining to tolerability.
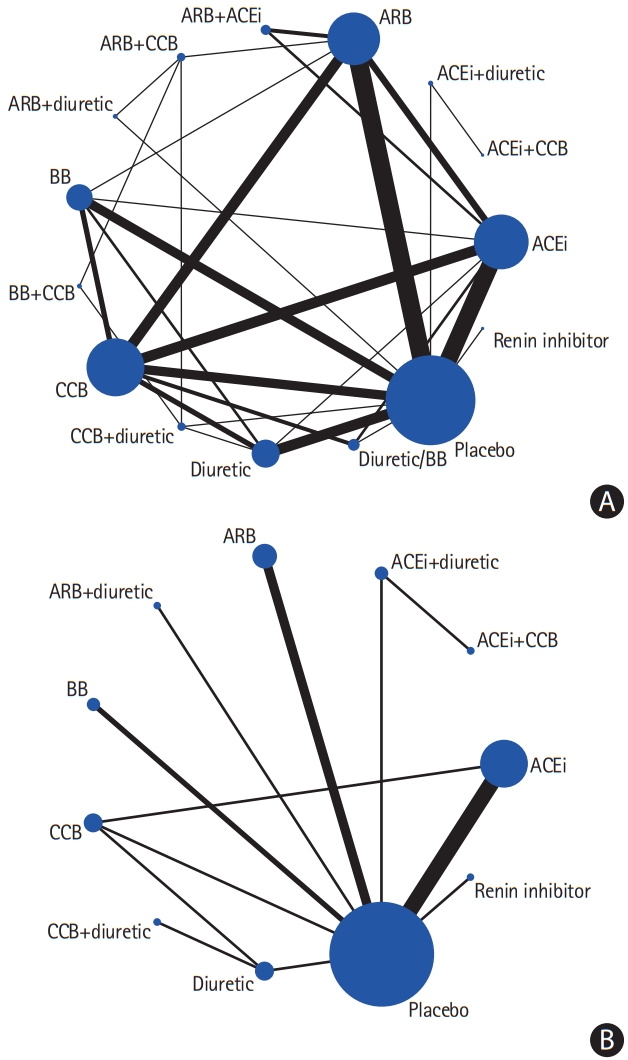
(A) Network of the studies included in the review with the available direct comparisons regarding efficacy. (B) Network of the studies included in the review with the available direct comparisons regarding tolerability. The width of the lines and the size of the nodes are proportional to the number of studies compared in each pair of treatments and the total sample size pertaining to each treatment, respectively. ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; CCB, calcium channel blocker; BB, beta-blocker.
Pairwise meta-analysis
The results of the pairwise meta-analysis for efficacy and tolerability profiles were summarized in Supplementary Table 2. Among the monotherapies, CCB and diuretics were associated with a reduced incidence of about one-third of strokes, while beta-blockers (BBs) and ACEis were associated with a risk reduction of 19% and 8%, respectively. Among the combination treatments, the combination of CCB+THZ was associated with a 30% reduction in stroke risk, compared to the placebo. An assessment of the comparative efficacy of different strategies revealed that ACEi and BB were inferior to CCB (ACEi vs. CCB [OR, 1.123; 95% CI, 1.008 to 1.252], BB vs. CCB [OR, 1.261; 95% CI, 1.116 to 1.425]), BB was inferior to ARB (OR, 1.361; 95% CI, 1.142 to 1.622), and diuretics were superior to ACEi (OR, 0.871; 95% CI, 0.771 to 0.984). Regarding the tolerability profile of monotherapies, ACEi was inferior to CCB (OR, 4.201; 95% CI, 2.206 to 7.998) and the combination of ACEi+CCB was superior to the combination of ACEi+THZs (OR, 0.892; 95% CI, 0.823 to 0.966).
Network meta-analysis-efficacy
The results of the network meta-analysis are shown in Figure 5. Compared to the placebos, all BP-lowering treatments, including CCB (OR, 0.74; 95% CI, 0.67 to 0.82), ARB (OR, 0.81; 95% CI, 0.73 to 0.89), ACEi (OR, 0.81; 95% CI, 0.73 to 0.90), diuretic (OR, 0.68; 95% CI, 0.59 to 0.77), CCB+THZ (OR, 0.71; 95% CI, 0.53 to 0.94), diuretic/BB (OR, 0.79; 95% CI, 0.65 to 0.94), and ARB+ACEi (OR, 0.78; 95% CI, 0.63 to 0.96), showed a benefit in stroke prevention. However, there was no correlation between the CCB+BB (OR, 1.38; 95% CI, 0.72 to 2.53) or the renin inhibitor (OR, 1.26; 95% CI, 0.85 to 1.79) strategy and stroke prevention. Diuretic use was superior to BB (OR, 1.34; 95% CI, 1.11 to 1.58), ACEi+THZ (OR, 1.47; 95% CI, 1.02 to 2.08), BB+CCB (OR, 2.05; 95% CI, 1.05 to 3.79), and renin inhibitor strategy (OR, 1.87; 95% CI, 1.25 to 2.75) in stroke prevention. The renin inhibitor was probably inferior to all the other treatments. Results from the pairwise meta-analysis and network meta-analysis for stroke prevention are summarized in Supplementary Table 3.
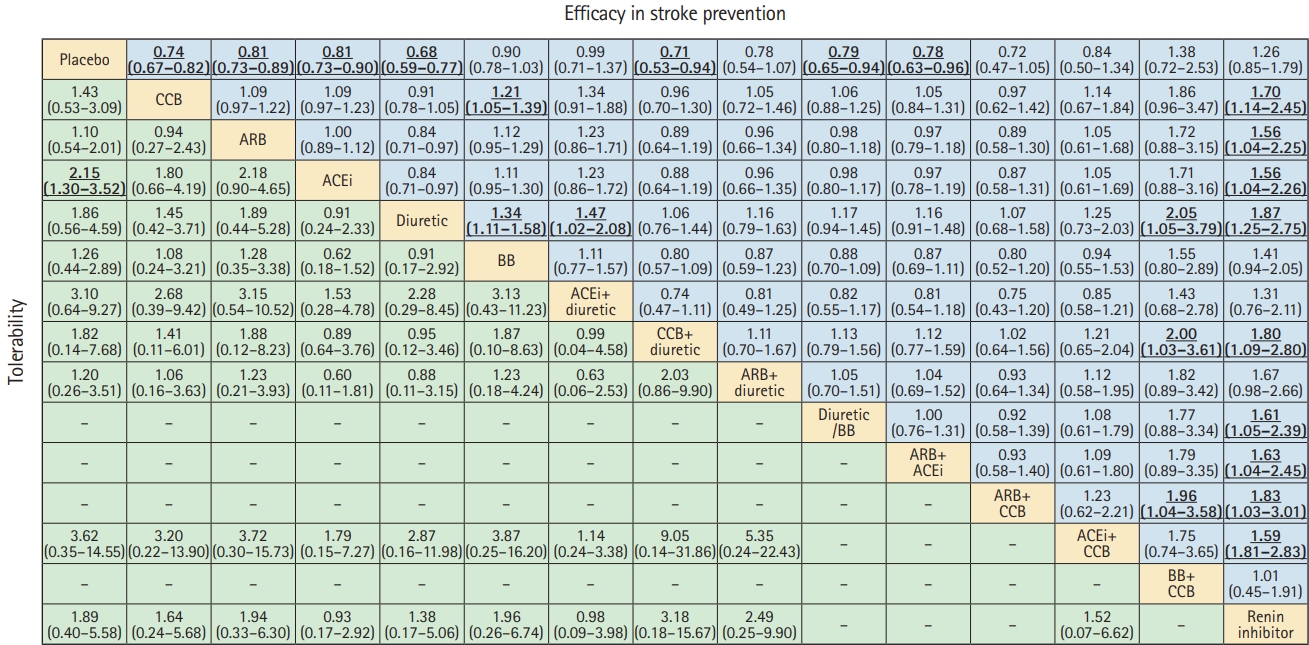
Comparative efficacy and tolerability of all blood pressure lowering treatments in stroke prevention, as per the network meta-analyses. Effect sizes represent summary odds ratios and 95% credible intervals. In the upper triangle (efficacy in stroke prevention), values greater than 1 favor the treatment in the corresponding row, whereas values less than 1 favor the treatment in the corresponding column. In the lower triangle (tolerability), values greater than 1 favor the treatment in the corresponding column, whereas values less than 1 favor the treatment in the corresponding row. Significant results are in bold and underlined. CCB, calcium channel blocker; ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; BB, beta-blocker.
The SUCRA values pertaining to the 15 different antihypertensive strategies were 88.4, 76.7, 74.8, 72.9, 62.5, 61.4, 59.2, 53.6, 53.1, 52.3, 32.9, 25.7, 17.9, 10.6, and 8.0 for diuretic, CCB+THZ, ARB+CCB, CCB, ARB+THZ, ARB+ACEi, diuretic/BB, ACEi+CCB, ARB, ACEi, BB, ACEi+THZ, placebo, BB+CCB, and renin inhibitor, respectively (Supplementary Figure 28). The mean rank associated with all the treatments is shown in Supplementary Figure 29. The cluster rank plot indicated that diuretics, CCB+THZ, CCB, ARB+THZ, and ARB were associated not only with a reduction in stroke risk, but also a lower rate of withdrawal, owing to drug-related side effects (Figure 6). Sensitivity analyses stratified by age (age ≤60 or >60 years), comorbidities (history of hypertension, stroke, diabetes, or heart failure), and baseline SBP (baseline SBP ≤150 or >150 mm Hg) showed robust results pertaining to the efficacy (Supplementary Table 4). Diuretics ranked first in most of the analyses. It is worth mentioning that the combination of ARB+CCB ranked first in the participants with baseline SBP above 150 mm Hg.

Cluster ranking for efficacy and tolerability of blood pressure lowering treatments in network meta-analyses. Each color represents a group of treatments that belong to the same cluster. Treatments located on the upper right corner are more effective and acceptable, compared to the other treatments. ARB, angiotensin II receptor blocker; BB, beta-blocker; CCB, calcium channel blocker; ACEi, angiotensin-converting enzyme inhibitor.
Network meta-analysis-tolerability
Network meta-analysis also confirmed that ACEi (OR, 2.15; 95% CI, 1.30 to 3.52) was likely to be associated with a significantly higher risk of withdrawal, owing to drug-related side effects compared to placebo. The comparative tolerability of different strategies is shown in Figure 5.
The SUCRA values for the 11 antihypertensive agents were 23.0, 24.3, 31.3, 40.4, 46.3, 53.6, 60.6, 61.9, 67.8, 68.4, and 72.5, for ACEi+THZ, ACEi, ACEi+CCB, diuretic, renin inhibitor, CCB, CCB+THZ, BB, ARB, ARB+THZ, and placebo, respectively (Supplementary Figure 30). The mean ranks pertaining to all the treatments is shown in Supplementary Figure 31. Visual inspection of funnel plots for efficacy did not show any distinct asymmetry (Supplementary Figure 32). However, several trials fell outside the significance boundaries in the tolerability analysis (Supplementary Figure 33), which could be attributed to the limited number of trials.
The assessment of transitivity is shown in Supplementary Figure 34. No inconsistency between direct and indirect estimates in node splitting was apparent, except for the two comparisons (placebo-ARB, CCB-ARB) on efficacy (Supplementary Table 5) and two nodes (placebo-ACEi, CCB-ACEi) on tolerability (Supplementary Table 6). Finally, the design-by-treatment inconsistency model was applied, and inconsistency for efficacy or tolerability was not detected in the current analyses (Supplementary Table 7).
Discussion
Using the data pertaining to 504,613 participants in 93 large RCTs, the current study has supplemented the information regarding the selection of the most appropriate antihypertensive agents for different populations with regard to the efficacy and tolerability, and confirmed that the reduction in BP was significantly associated with lower mortality rates and stroke incidence. The therapeutic benefits existed regardless of the stratification by comorbidities, age, and baseline SBP. All the achieved SBP levels were associated with a 15% to 45% stroke risk reduction. An interesting, but unexpected finding in the current study was that diuretics were more effective in stroke prevention, compared to the other BP-lowering treatments. CCB+THZs, CCB, ARB+THZs, and ARB were also appropriate options in terms of efficacy and tolerability.
The observations in the present study are concurrent with the previously published meta-analyses with reference to the target SBP [3,21]. Moreover, the present study supplemented the information that BP-lowering was significantly associated with the reduced stroke incidence in all subtypes including ischemic, hemorrhagic, and fatal or disabling stroke. The current study showed that lowering BP to a target of below 130 mm Hg could reduce the risk associated with stroke. However, this should be interpreted with caution. Among the ischemic stroke patients without intracranial artery stenosis (lacunar infarction), intensive lowering of BP to a target of below 130 mm Hg is more beneficial in the reduction of intracerebral hemorrhage, rather than risk of ischemic stroke [22]. Moreover, intensive BP-lowering in patients with intracranial atherosclerotic stenosis may increase the ischemic lesion volume in the subacute stage [23]. Hence, intensive BP control to a target of below 130 mm Hg should mainly be recommended for the primary prevention of both ischemic and hemorrhagic strokes. The results of the present study, confirmed that a changed in target SBP from 140 to 130 mm Hg was necessary [2,3]. The target SBP for antihypertensive treatment is controversial, especially for the populations in different age groups. The guidelines of the Eighth Joint National Committee (JNC8) (2014) recommend a goal of BP below 140/90 mm Hg in patients younger than 60 years and a more relaxed goal of below 150/90 mm Hg in those older than 60 years [24]. Another meta-analysis explored the benefits and harms of intensive BP management in adults aged 60 years or above and found that the treatment with a target BP below 150 mm Hg improves the health outcomes including stroke in older adults and lower targets (≤140/85 mm Hg) are associated with a marginally significant decrease in stroke incidence [25].
In the present study, the network meta-analysis suggested that diuretics, CCB, ARB, ACEi, and all diuretic-based combination therapies were effective in stroke prevention. This was consistent with the 2017 high BP guideline, which observed that no class of antihypertensive medications were better than THZs in reducing the risk of stroke and various cardiovascular outcomes [5]. However, the present study differs from the 2017 high BP guideline in some aspects. For instance, the 2017 high BP guideline excluded placebo-controlled trials, whereas the current study included the trials that compared BP-lowering agents to placebo controls. Moreover, the 2017 high BP guideline examined only the first-line antihypertensive medications including diuretics, ACEis, ARBs, CCBs, and BBs, whereas the current study examined all the available antihypertensive medications.
Diuretics have been preferred as the first-line antihypertensive agents since the release of the results of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), which suggested that diuretics were as effective as CCBs in reducing specified endpoints [26]. However, a previous meta-analysis explored the efficacy of CCBs and other antihypertensive agents and reported that there was no significant difference between CCBs and other comparators with regard to the efficacy [6]. the present analysis included the trials published in or after 2000 and suggested that CCB+THZs is the most effective therapy for the reduction of stroke incidence, followed by ARB+CCB, diuretics, and ARB+diuretics. In view of these findings, CCB, ARB, and diuretics could be employed as the firstline drugs for stroke prevention. However, in view of the adverse effects of diuretics, especially at high dosages, caution should be exercised when prescribing to populations with an increased risk of developing diabetes and gout. In the aforementioned patients, monotherapy using CCBs and ARBs or combinations with diuretic therapies might be appropriate alternatives.
Thus far, no network meta-analysis has been performed to examine the efficacy and tolerability of various antihypertensive agents for stroke prevention in the entire population. A previous network meta-analysis studied the various antihypertensive agents for stroke prevention in patients with type 2 diabetes and concluded that none of the antihypertensive agents were superior to one another, including the placebos [27]. The current study demonstrated that ACEi-based single or combination therapies were most likely to be associated with the withdrawal, owing to drug-related side effects, whereas ARB-based single or combination therapies were well tolerated. This advantage might be helpful in the decision-making process when balancing the efficacy and feasibility.
The current study has certain limitations. First, a relatively small number of trials exploring the effects of antihypertensive agents for secondary stroke prevention were included, which precluded the execution of a formal network meta-analysis to determine the relative efficacy of different antihypertensive therapies for secondary stroke prevention. Second, the transitivity assumption during the network analysis was unavoidable. Many RCTs included in the present analysis involved combination therapies and the inclusion of combination therapies in network meta-analysis of first-line treatments would introduce intransitivity [28]. Third, the concurrent discretionary use of statins, dual anti-platelets therapy, stringent glycemic control by new diabetes drugs, lifestyle coaching, and the ‘add-on’ antihypertensive drugs allowed in the recent/newer trials might diminish the marginal benefit of the new classes of antihypertensive drugs. Fourth, the present study failed to acquire the relevant data from studies involving diabetic subgroups, stroke subtypes, reason for withdrawal, the elapsed time between initiation of antihypertensive agents and the index stroke, as most of these studies were not included. Fifth, as the present review includes the studies that were published over a long period of time (1966–2018), the definition of stroke and the incorporation of the advances in neuroimaging can be considered to be different among the trials. Sixth, considering the ageing population, an average duration of follow-up of 3.3 years remains limited and trials with longer follow-up periods are warranted. Lastly, the trials included in the current study varied in several aspects, including the study population, race, baseline characteristics, study methodology, and concurrent use of multiple classes of antihypertensive drug. Consequently, the possibility that the differences between treatment strategies attributed to the aforementioned biases could not be excluded. The present study attempted to minimize the heterogeneity by performing sensitivity and subgroup analyses, in order to provide more robust conclusions.
In conclusion, the BP-lowering strategy is significantly associated with the risk reduction of all-cause mortality, cardiovascular-related death, all stroke types (fatal or nonfatal), disabling stroke, ischemic stroke, and hemorrhagic stroke. Monotherapy with diuretics, CCB or ARB, and their combinations could be employed as the first-line treatments for stroke prevention in terms of the efficacy and tolerability. Relatively, ACEi has a higher risk of side effect-related withdrawal.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.02698.
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2018YFC1314700), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of the Ministry of Education, Fudan University, and Qingdao Municipal Medical Research Guidance Program (2019-WJZD082).