 |
 |
- Search
| J Stroke > Volume 22(2); 2020 > Article |
|
Dear Sir:
Multiple multicenter randomized clinical trials have established mechanical thrombectomy (MT) as the standard of care (SOC) for large vessel occlusion (LVO) acute ischemic strokes (AIS) [1]. It is also established quite definitely that in the early thrombectomy window (0 to 6 hours), the neurological functional outcome is highly time-sensitive and designing workflow to minimize delays of even few minutes is critical [2]. Currently, more than 1 million cardiac catheterization (CC) procedures are performed annually in the United States. The reported incidence of acute stroke during CC in the literature ranges from 0.1% to 0.6% [3] and if a LVO stroke, can result in lifelong disability. Use of intravenous recombinant tissue plasminogen activator (IV-rtPA) therapy for AIS during CC is not well established with concern for relatively higher risk of bleeding due to procedural anticoagulation. With arterial access already in place, an immediate “direct” cerebral digital subtraction angiogram (cDSA) can expedite a definitive diagnosis of LVO allowing rapid MT in such patients. Finding a LVO on immediate cDSA in a patient with acute stroke syndrome during CC makes the possibility of concurrent intracerebral hemorrhage (ICH) highly unlikely as a cause of the syndrome. A conventional head computed tomography (CT) necessitates moving the patient from the CC laboratory table to CT and then to neuro-angiography suite which adds significant delay to reperfusion benefit.
We hypothesized that a novel triage strategy of “direct” cDSA, bypassing conventional head CT, followed by MT in patients who suffered a LVO AIS during CC procedures is safe and would keep the onset-to-reperfusion times short. “Direct” is defined as performing cDSA for MT in the same single-plane CC suite foregoing conventional CT to rule out ICH. In this case series we report two large tertiary-care comprehensive stroke centers experience with this management strategy, which was agreed upon by both cardiology and neuro-endovascular teams. All direct-cDSA and MT procedures were performed by Endovascular Surgical Neuroradiology fellowship-trained neuro-interventionalists. Figure 1 highlights our institutional workflow algorithm for direct-cDSA.
Four consecutive cases were included in this case series from January 2016 to June 2017, where the acute focal neurological syndrome was identified while patients were still in the CC suite and had the arterial sheath in place. Table 1 details the baseline characteristics and covariates of patients included in this case series. All patients identified were undergoing emergent CC, three for NSTEMI and one for unstable angina. A CT brain was obtained within 24-hour post-MT to evaluate for any hemorrhagic transformation (HT) of the infarcted cerebral tissue. LVO was confirmed in three out of four patients who subsequently underwent successful MT (Figure 2). One patient had patent vessels on direct-cDSA with rapid improvement in symptoms suggesting spontaneous recanalization or non-LVO stroke. Immediate head CT obtained in this patient did not show an ICH, and IV-rtPA was not administered because symptoms spontaneously improved with return of National Institutes of Health Stroke Scale to 0. In aggregate, the mean times from the identification of neurological deficits to start of cDSA procedure, first pass and successful revascularization was 17, 43 and 54 minutes, respectively. In all cases, the same transfemoral arterial access (TFA) obtained by a cardiologist was utilized for initial cDSA with subsequent upsizing of the access sheath to 8 Fr as needed for MT. Anticoagulation with intravenous unfractionated heparin during CC procedures was used in three of four patients. IV-rtPA was not administered in any case. All CC procedures were performed under monitored anesthesia care, and none of the patients required conversion to general anesthesia for MT. Solitaire stent retriever with balloon guide catheter and standard endovascular stroke techniques were used for all MT procedures.
Since MT became SOC in 2015 and the outcomes were found to be highly time sensitive [1], a major focus is to devise time saving workflow strategies to maximize good outcomes. Our case series indicate that if a LVO is seen on cDSA in the vascular territory referable to the stroke syndrome occurring during CC, the need for a CT to rule out hemorrhage appears to be redundant. None of the patients in our series had a HT on subsequent CT brain indicating safety of our approach. There is anecdotal evidence documenting the safety of reversal of procedural anticoagulation with IV heparin with protamine followed by successful administration of IV-rtPA for AIS during CC [4]; but as there is no clear consensus on this approach we opted to forego the same.
In keeping with the trend of preferred increased use of trans-radial access for cardiovascular procedures, it is important to highlight the fact that successful MT can be performed using the same radial access which can be upsized to 6 Fr as needed, without the need for crossover to TFA [5]. Furthermore, the angiographic suites equipped with the newer flat-panel detector technology have the capability of obtaining a sufficient quality DynaCT (Siemens Medical Solutions, Erlangen, Germany), which can be used to rule out ICH [6] and if negative, would obviate the need to remove the patient from angiography table supporting our approach for direct-MT.
Our case series suggests that performing immediate direct cDSA followed by MT without conventional CT is feasible, safe and keeps the stroke onset-to-reperfusion times very short in patients with suspected LVO during CC procedures. Therefore, further and larger studies are needed to prove the use of this approach in routine clinical practice.
Notes
Dr. Yavagal is on the steering committee of TIGER clinical trial sponsored by Rapid Medical and steering committee of CALM-2 sponsored by Vascular Dynamics. He is a consultant to Medtronic, Cerenovus, Poseydon, Neurosave, and other Neuralanalytics. All of these relationships are outside of the current work.
Figure 1.
Algorithm to identify patients with neurological deterioration during cardiac catheterization who would benefit from direct cerebral angiogram and mechanical thrombectomy. NIHSS, National Institutes of Health Stroke Scale; NIR, neuro-interventional radiology; LVO, large vessel occlusion; CT, computed tomography; MRI, magnetic resonance imaging; tPA, tissue plasminogen activator. *Focal neurological deficits were defined as focal motor weakness or cortical deficits such as horizontal gaze deviation, hemi-visual or sensory neglect, aphasia or hemianopsia; †If cardiac catheterization suite is equipped with the newer flat-panel detector technology, a sufficient quality DynaCT (Siemens Medical Solutions) can be obtained to rule out intracranial hemorrhage, and if negative, neurointerventional team can proceed with a direct-cerebral digital subtraction angiography. This technology was not available in our cardiac catheterization suite at the time these procedures were performed; ‡Cases discussed with both cardiology and neurology teams to weigh the risk and benefit of completing cardiac catheterization procedure before or after obtaining further cerebral imaging such as CT or MRI brain as needed; §tPA can be considered after intracranial
hemorrhage is ruled out and if coagulation lab tests such as prothrombin time-international normalized ratio and partial thromboplastin time are permissive for intravenous thrombolytic use per institutional guidelines.
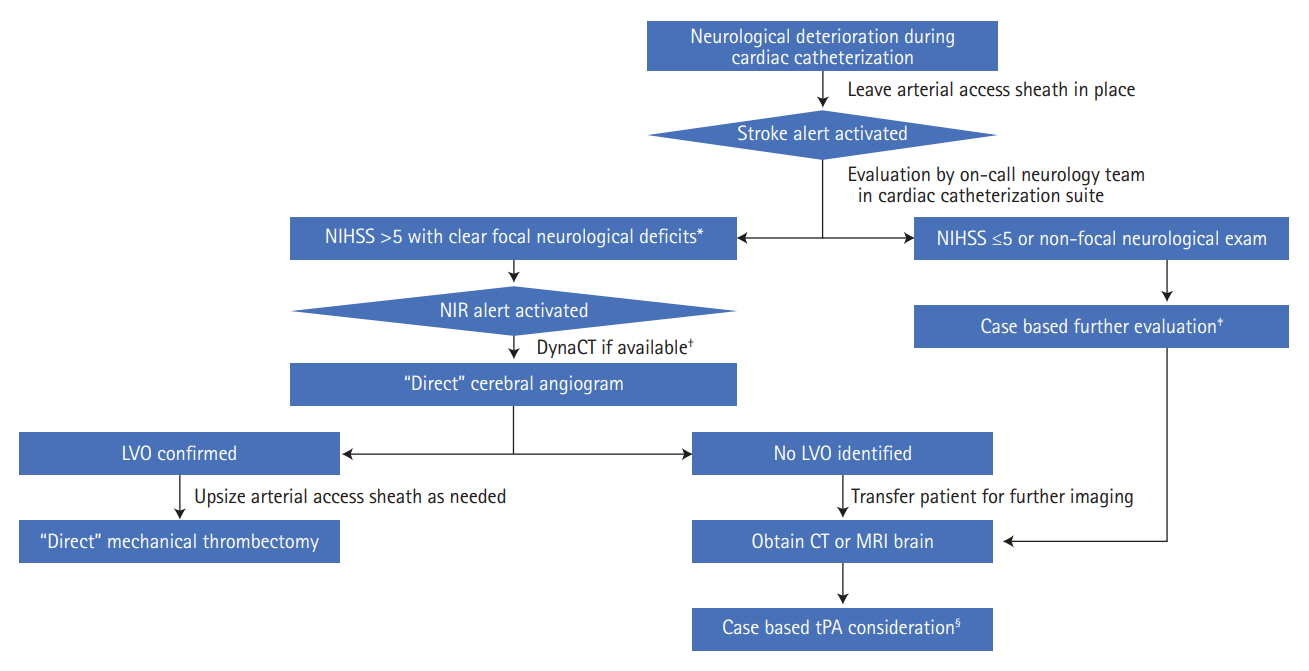
Figure 2.
(A, B) Pre- and post-mechanical thrombectomy (MT) catheter cerebral angiogram images performed in the single-plane cardiac catheterization suite. (C, D). Initial diagnostic cardiac catheter angiogram on admission and percutaneous coronary intervention with a drug-eluting stent placement in left main coronary artery performed on day 6 post-MT. Case 1 (refer Table 1) underwent initial cardiac catheter angiogram showing left main coronary artery >90% stenosis (C; white arrow), complicated by near-complete left middle cerebral artery (MCA) syndrome, and underwent immediate direct cerebral angiogram showing proximal second division of MCA respectively (M2) cut-off (A; white arrow). Direct-MT performed with successful thrombolysis in cerebral infarction 3 reperfusion (B). On day 6 post-MT patient underwent successful percutaneous coronary intervention of left main coronary artery and ostial/proximal left circumflex with drug-eluting stent placement with resulting thrombolysis in myocardial infarction 3 flow.

Table 1.
Baseline patient characteristics, procedural and outcome details of catheter cerebral angiography and mechanical thrombectomy
| Age (yr), sex | Clot location and clinical syndrome | Onset to cDSA start (min)* | Onset to first pass (min)* | Onset to recanalization (min)* | TICI grade† | No. of passes | Initial NIHSS | 2-hr post-MT NIHSS | mRS at discharge | Indication for cardiac | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 63, F | L M2; near-complete L MCA syndrome‡ | 15 | 25 | 31 | 3 | 1 | 21 | 2 | 1 | Unstable angina/NSTEMI; 1 coronary DES placed on day 6 post-MT |
| Case 2 | 69, F | L M1; complete L MCA syndrome | 26 | 41 | 46 | 3 | 1 | 26 | 11 | 2 | NSTEMI; no coronary stent placed |
| Case 3 | 70, F | R M2; complete R MCA syndrome | 17 | 62 | 85 | 2b | 1 | 25 | 18 | 3 | NSTEMI; 2 coronary DES placed prior to the neurological deficit |
| Case 4§ | 50, F | Suspected R M1; near-complete R MCA syndrome | 11 | NA | NA | NA | NA | 18 | 0 | 0 | NSTEMI; no coronary stent placed |
cDSA, cerebral digital subtraction angiography; TICI, thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; MT, mechanical thrombectomy; mRS, modified Rankin Scale; L, left; M1,2, sequential divisions of MCA; MCA, middle cerebral artery; NSTEMI, non-ST elevation myocardial infarction; DES, drug-eluting stent; R, right; NA, not applicable.
* Onset time was defined as the time neurological deterioration was noted by cardiac catheterization team. As the arterial access sheath was already in place, time to start of cDSA procedure was defined as the first angiographic run by neurointerventional team. First pass was defined as the first attempt at clot retrieval during MT. The final angiographic run confirming the patency of previously occluded vessel after successful MT was documented as the recanalization time;
‡ Complete MCA syndrome is defined as contralateral hemiplegia and hemisensory loss, ipsilateral horizontal gaze deviation, aphasia or dysarthria (in patients with involvement of dominant or non-dominant hemisphere respectively), contralateral hemianopsia and contralateral hemi-visual and/or hemi-sensory neglect (in patients with non-dominant hemisphere involvement). The severity of the syndrome is defined by NIHSS score (range 0-42);
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-1731.


3. Werner N, Bauer T, Hochadel M, Zahn R, Weidinger F, Marco J, et al. Incidence and clinical impact of stroke complicating percutaneous coronary intervention: results of the Euro heart survey percutaneous coronary interventions registry. Circ Cardiovasc Interv 2013;6:362-369.


4. Warner DS, Schwartz BG, Babygirija R, Rovin RA, Kassam AB, Biddick L, et al. Thrombolysis after protamine reversal of heparin for acute ischemic stroke after cardiac catheterization: case report and literature review. Neurologist 2018;23:194-196.









