Association of MicroRNA Biogenesis Genes Polymorphisms with Ischemic Stroke Susceptibility and Post-Stroke Mortality
Article information
Abstract
Background and Purpose
MicroRNA (miRNA) expression has been examined in multiple conditions, including various cancers, neurological diseases, and cerebrovascular diseases, particularly stroke. Existing evidence indicates that miRNA biosynthesis and function play crucial roles in ischemic stroke physiology and pathology. In this study, we selected six known polymorphisms in miRNA-biogenesis genes; DICER rs13078A>T, rs3742330A>G; DROSHA rs10719T>C, rs6877842G>C; Ran GTPase (RAN) rs14035C>T; exportin 5 (XPO5) rs11077A>C.
Methods
We analyzed the associations between these polymorphisms and disease status and clinical factors in 585 ischemic stroke patients and 403 controls. Genotyping was performed with the polymerase chain reaction-restriction fragment length polymorphism method.
Results
The DICER rs3742330A>G (AA vs. AG+GG: adjusted odds ratio [AOR], 1.360; 95% confidence interval [CI], 1.024 to 1.807; P=0.034) and DROSHA rs10719T>C polymorphisms (TT vs. CC: AOR, 2.038; 95% CI, 1.113 to 3.730; P=0.021) were associated with ischemic stroke prevalence. During a mean follow-up of 4.80±2.11 years, 99 (5.91%) of the stroke patients died. In multivariate Cox proportional hazard regression models, a significant association was found between RAN rs14035 and survival of large artery disease patients with ischemic stroke (CC vs. TT: adjusted hazard ratio, 5.978; P=0.015).
Conclusions
An association was identified between the DICER and DROSHA polymorphisms and ischemic stroke. Specifically, polymorphisms (rs3742330 and rs10719) were more common in stroke patients, suggesting that they may be associated with an increased risk of ischemic stroke.
Introduction
Stroke is regarded as a complex, multifactorial, polygenic disease arising from a wide number of gene-gene and gene-environment interactions [1,2]. Multiple factors including hypertension, diabetes mellitus, smoking, hyperlipidemia, and hyperhomocysteinemia are associated with a higher risk of stroke [3,4]. Hyperhomocysteinemia, in particular, has been demonstrated to be an independent risk factor for ischemic stroke in several studies involving different ethnic groups [5,6].
MicroRNAs (miRNAs) are a class of endogenous, small, noncoding RNAs that pair with sites in 3’-untranslated regions (3’-UTRs) in mRNAs to downregulate their expression [7-9]. Previous studies have suggested that gene expression may be regulated by a small number of miRNAs [10-13]. To date, miRNA expression has been examined in patients with tumors [12,14,15], Alzheimer’s disease [16], Parkinson’s disease [17], schizophrenia [18], and stroke [19-21]. The evidence gathered to date indicates that miRNA biosynthesis plays crucial, physiological, and pathological roles [22-24]. Biosynthesis of miRNAs involves several miRNA biogenesis genes and occurs in multiple steps [7]. RNA polymerase II produces large primary miRNA transcripts (about 500 to 3,000 nucleotides) in the nucleus. The transcripts are processed by a multiprotein complex that includes DROSHA to form precursor miRNA (pre-miRNA) hairpins (about 60 to 100 nucleotides). After pre-miRNA has been exported to the cytoplasm by Ran GTPase (RAN) and exportin 5 (XPO5), it is further processed by DICER1, a polymerase II enzyme. Subsequently, the double-stranded miRNA duplex unwinds, forming an 18- to 24-nucleotide single-stranded, mature miRNA [7,25-27].
The present study tested the hypothesis that there is an association between miRNA biogenesis gene polymorphism and ischemic stroke risk. The objective was to investigate associations between six known miRNA biogenesis gene polymorphisms (DICER 3’-UTR rs13078A>T, DICER 3’-UTR rs3742330A>G, DROSHA 3’-UTR rs10719T>C, DROSHA 3’-UTR rs6877842G>C, RAN 3’-UTR rs14035C>T, and XPO5 3’-UTR rs11077A>C) and ischemic stroke and its risk factors.
Methods
Ethics statement
All study protocols were reviewed and approved by the Institutional Review Board of CHA Bundang Medical Center and followed the recommendations of the Declaration of Helsinki. Study subjects were recruited from the South Korean provinces of Seoul and Gyeonggi-do between 2000 and 2008. The Institutional Review Board of CHA Bundang Medical Center approved this genetic study in June 2000 (IRB No. 2013-09-073) and informed consent was obtained from study participants.
Study population
The Department of Neurology at CHA Bundang Medical Center, CHA University, referred 585 consecutive patients with ischemic stroke. Ischemic stroke was defined as a stroke (a clinical syndrome characterized by rapidly developing clinical symptoms and signs of focal or global loss of brain function) with evidence of cerebral infarction in clinically relevant areas of the brain according to magnetic resonance imaging (MRI) scan finding. Based on clinical manifestations and neuroimaging data, two neurologists classified all ischemic strokes into four causative subtypes using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, as follows: (1) large artery disease (LAD), characterized by an infarction lesion ≥15 mm in diameter documented by MRI, and significant (>50%) stenosis of a major brain artery or a branch cortical artery documented by cerebral angiography with symptoms associated with that arterial territory; (2) small vessel disease (SVD), characterized by an infarction lesion <15 and ≥5 mm in diameter documented using MRI, and classic lacunar syndrome without evidence of cerebral cortical dysfunction or potentially detectable cardiac sources for embolism; (3) cardioembolism (CE) or arterial occlusions presumably due to an embolus arising in the heart, as detected by cardiac evaluation; and (4) undetermined pathogenesis, in which the cause of stroke could not be determined with any degree of confidence or involved >2 causes. Single and multiple (≥2 lesions) SVD cases were distinguished via brain MRI scans. The sizes and sites of cerebral infarctions were documented using MRI only. We selected 403 control subjects that were matched for sex ratio and age (within 5 years) in accordance with the patient group (Table 1). Controls were drawn from subjects visiting our hospitals during the same period for health examinations, including biochemical testing, electrocardiograms, and brain MRIs. Control subjects did not have a recent history of cerebrovascular disease or myocardial infarction. Exclusion criteria were the same as those used for the case group, as mentioned previously.
Genotyping
DNA was extracted from leukocytes using a G-DEX II Genomic DNA Extraction kit (Intron Biotechnology, Seongnam, Korea) according to the manufacturer’s instructions. The six best-studied single nucleotide polymorphisms (SNPs) in the miRNA biogenesis genes were determined through a documentary search that included 3′-UTR SNPs (DICER rs13078A>T, rs3742330A>G, DROSHA rs10719T>C, rs6877842G>C, RAN rs14035C>T, and XPO5 rs11077A>C). The miRNA biogenesis gene polymorphisms were analyzed by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The PCR conditions for miRNA biogenesis genes polymorphism analyses are presented in Supplementary Table 1. To validate RFLP findings, 30% of the PCR assays for each polymorphism were randomly selected and repeated, followed by DNA sequencing. Sequencing was performed using an ABI 3730×l DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The concordance of these quality control samples with the RFLP results was 100%.
Post-stroke mortality
To evaluate the association between miRNA biogenesis gene polymorphisms and long-term prognosis after ischemic stroke, survival time from stroke onset to death was tracked. The dates of death for each stroke patient (n=585) were ascertained using death certificates from the Korean National Statistical Office. Patients who were alive on December 31, 2013 were excluded from the study.
Statistical analysis
Genotype and allele combination frequencies in ischemic stroke cases and controls were compared using multivariate logistic regression models and Fisher exact test, respectively. Allele frequencies were calculated to identify deviations from Hardy-Weinberg equilibrium using P=0.05 as a threshold. Odds ratios, adjusted odds ratios (AORs), and 95% confidence intervals (CIs) were used to measure the strength of association between various genotypes and ischemic stroke. The association between miRNA biogenesis gene SNPs and post-stroke mortality was evaluated using Cox proportional hazard regression. The proportional hazards assumption was tested using a log(–log[survival]) plot and interaction for follow-up time in a time-dependent Cox regression model, which was found to be satisfactory. For multivariate analyses, logistic regression analyses were used to adjust for possible confounders, including age, sex, hypertension, diabetes mellitus, hyperlipidemia, and smoking. Statistical significance was accepted at the P<0.05 level [28,29].
Results
Baseline characteristics
The demographic characteristics of the 585 stroke cases and 403 controls are presented in Table 1. Of the stroke and control samples, 41.5% and 41.7%, respectively, were men, and the mean ages of stroke cases and controls were 62.7±10.9 and 62.8±10.6 years, respectively.
Genotype frequencies of the miRNA biogenesis genes polymorphisms
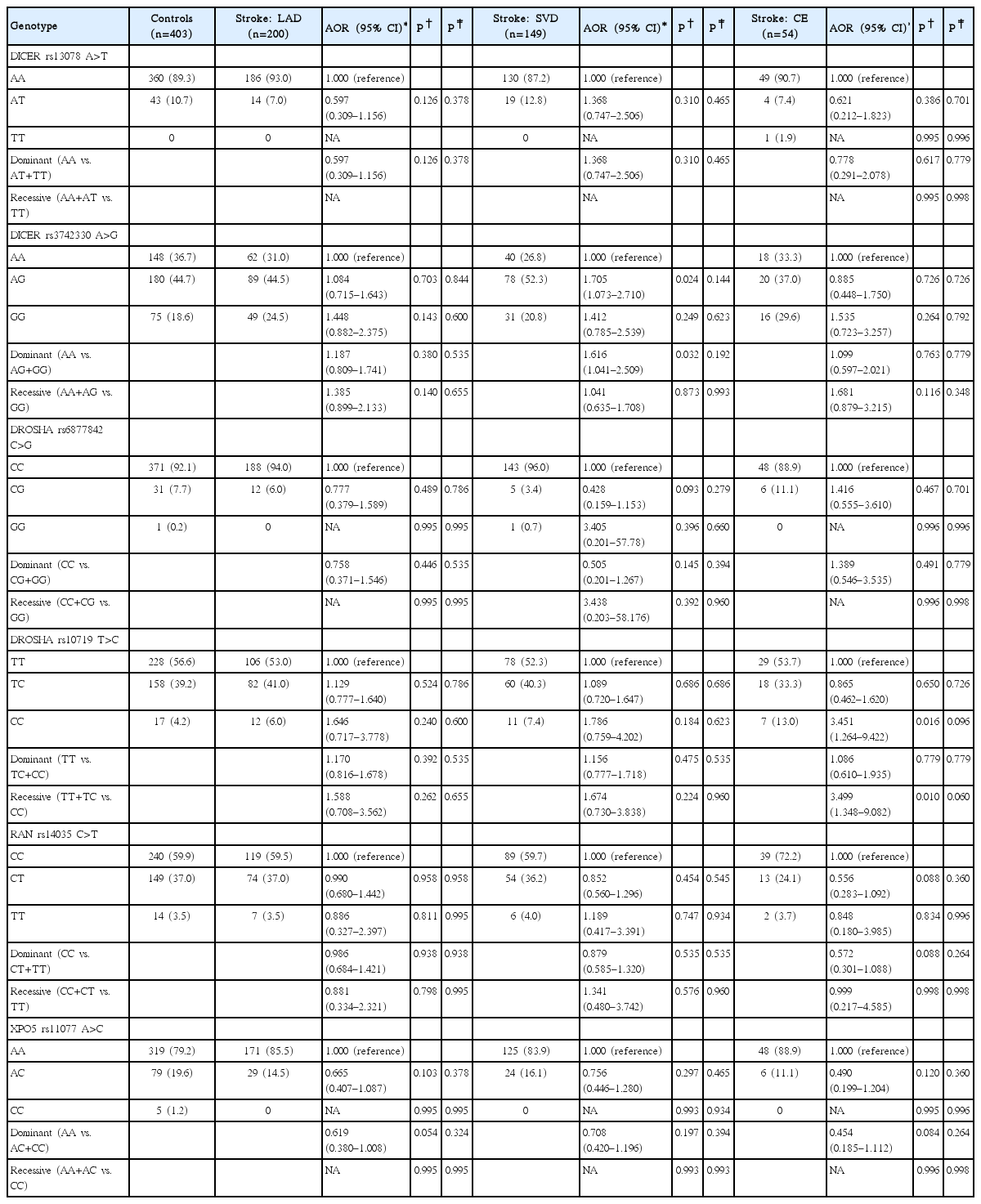
Table 2 provides the genotype distributions of the six miRNA biogenesis gene polymorphisms in ischemic stroke cases and controls. The DICER rs3742330A>G polymorphism was associated with greater odds of ischemic stroke (AA vs. GG: AOR, 1.459; 95% CI, 1.000 to 2.126; P=0.050; and AA vs. AG+GG: AOR, 1.360; 95% CI, 1.024 to 1.807; P=0.034). The DROSHA rs10719T>C polymorphism was also associated with greater odds of ischemic stroke (TT vs. CC: AOR, 2.038; 95% CI, 1.113 to 3.730; P=0.021; and TT+TC vs. CC: AOR, 2.001; 95% CI, 1.106 to 3.621; P=0.022). By contrast, the XPO5 rs11077A>C polymorphism was associated with lower odds of stroke (AA vs. CC: AOR, 0.101; 95% CI, 0.011 to 0.951; P=0.045; and AA vs. AC+CC: AOR, 0.669; 95% CI, 0.473 to 0.945; P=0.023). The frequency of the DICER1 rs13078A>T, DROSHA ABI 3730×l DNA Analyzer C>G, and RAN rs14035C>T polymorphisms was not significantly different between stroke cases and controls. To examine whether the effect of each polymorphism was confined to a specific subtype, stroke patients were separated into three subgroups (LAD, SVD, and CE) according to TOAST classifications (Table 3). Comparisons were also performed with control subjects and single versus multiple SVD patients (Supplementary Table 2). LAD was not significantly associated with any of the polymorphisms examined. However, the DICER1 rs3742330A>G polymorphism was significantly associated with SVD (AA vs. AG: AOR, 1.705; 95% CI, 1.073 to 2.710; P=0.024; and AA vs. AG+GG: AOR, 1.616; 95% CI, 1.041 to 2.509; P=0.032). In addition, the DROSHA rs10719T>C polymorphism was significantly associated with CE (TT vs. CC: AOR, 3.451; 95% CI, 1.264 to 9.422; P=0.016; and TT+TC vs. CC: AOR, 3.499; 95% CI, 1.348 to 9.082; P=0.010).

Comparison of DICER, DROSHA, RAN, and XPO5 polymorphisms between ischemic stroke patients and controls subjects
Combined effects of miRNA biogenesis gene polymorphisms and clinical factors
Stratified analysis of each clinical factor was performed to confirm the influence of clinical factors on the occurrence of ischemic stroke. However, no significant clinical factors were found to affect ischemic stroke risk (Supplementary Table 3). Therefore, a combined effect analysis was conducted to ascertain the effect of stroke and genotype on the prevalence of ischemic stroke. A synergistic effect was found for ischemic stroke prevalence between clinical factors (hypertension and diabetes mellitus) and miRNA biogenesis gene polymorphisms (Figure 1). The DROSHA rs10719 CC genotype was associated with stroke in individuals with hypertension (AOR, 4.781; 95% CI, 1.981 to 11.54). Diabetes mellitus combined with the DROSHA rs10719 CC genotype yielded the most significant association with stroke (AOR, 12.05; 95% CI, 1.541 to 94.19). Other gene-clinical factor combinations were not significantly associated with ischemic stroke (Supplementary Tables 4-6). The effects of miRNA biogenesis gene genotypes on blood coagulation status were evaluated by measuring platelet proportion, prothrombin time, activated partial thromboplastin time (aPTT), fibrinogen, and antithrombin (Supplementary Table 7). It was found that the DROSHA rs10719 CC genotype was significantly associated with elevated aPTT (TT vs. CC: P=0.007; TC vs. CC: P=0.019) (Supplementary Figure 1A) and antithrombin (TC vs. CC: P=0.039) (Supplementary Figure 1B). The other coagulant factors did not exhibit any statistically significant associations with any of the tested genotypes.

The effects of DROSHA rs10719 T>C variant on ischemic stroke development modulated by clinical factors. (A) The synergistic effect for ischemic stroke susceptibility in DROSHA rs10719CC with hypertension (adjusted odds ratio [AOR], 4.781), DROSHA rs10719CC with non-hypertension (AOR, 1.967), or DROSHA rs10719TT with hypertension (AOR, 2.415). (B) DROSHA rs10719 T>C was associated with elevated ischemic stroke prevalence, in the case of DROSHA rs10719CC, with diabetes mellitus (AOR, 12.046), DROSHA rs10719CC, with non-diabetes mellitus (AOR, 1.770), and DROSHA rs10719TT, with diabetes mellitus (AOR, 2.351).
Polymorphisms in miRNA biogenesis genes versus post-stroke mortality
To evaluate the association between miRNA biogenesis gene polymorphisms and post-stroke mortality, Cox regression analysis was performed on the 585 patients with total ischemic stroke according to TOAST subtype (Figure 2 and Supplementary Table 8). During a mean follow-up of 4.80±2.11 years, 99 of the stroke patients died. In the multivariate Cox proportional hazard regression models, a significant association was found between RAN rs14035 and survival of LAD patients with ischemic stroke (CC vs. TT: adjusted hazard ratio [HR], 5.978; P=0.015; and CC+CT vs. TT: adjusted HR, 3.946; P=0.034) (Figure 2). A significant association was also found between RAN rs14035 and SVD in our analysis of ischemic stroke subtypes (CC vs. TT: adjusted HR, 9.403; P=0.015; and CC+CT vs. TT: adjusted HR, 5.223; P=0.039) (Figure 2). However, survival analysis was performed by Cox proportional-hazards regression based on the stepwise method for confirming covariant effect. A stepwise Cox regression analysis of ischemic stroke-related survival is shown in Supplementary Table 9. Mortality in SVD subgroup of ischemic stroke cases was associated with age and RAN rs14039 polymorphism status.

Survival plot from a Cox proportional hazards model with RAN rs14035C>T polymorphisms in ischemic stroke. Survival curve of patients grouped by large artery disease (LAD) subtype based on (A) RAN rs14035CC vs. RAN rs14035TT genotypes and (B) RAN rs14035CC+CT vs. RAN rs14035TT genotypes. In addition, survival curve of patients grouped by SVD subtype based on (C) RAN rs14035CC vs. RAN rs14035TT genotypes and (D) RAN rs14035CC+CT vs. RAN rs14035TT genotypes. HR, hazard ratio.
Supplemental data
Gene-gene interaction analyses were performed for miRNA biogenesis gene polymorphisms to identify combinations that have synergistic effects on stroke risk (Supplementary Table 10 and 11). Some variants and allele combinations exhibited significant associations. However, the meaning of these associations should be interpreted with caution because the sample size is rather small.
Discussion
A recent study indicated that miR-221 and miR-222 modulate the angiogenic properties of human umbilical vein endothelial cells [30]. However, the function and biosynthesis of miRNAs in endothelial cell biology remains unclear. Therefore, in this study of ischemic stroke, we focused on miRNA biogenesis genes, such as DICER1, DROSHA, XPO5, and RAN, which are related to endothelial miRNA expression and angiogenesis [31,32]. We evaluated six polymorphisms in DICER1, DROSHA, XPO5, and RAN genes essential for miRNA biosynthesis [33-35], in ischemic stroke cases and controls. We found that polymorphisms in DICER and DROSHA, both of which are involved in angiogenesis and coagulation mechanisms [36-38], were linked to ischemic stroke. Polymorphisms in DICER, a gene already known to play a role in vascular growth and genesis, displayed the strongest association with ischemic stroke. For example, the DICER rs3742330 GG genotype was significantly more frequent in both overall stroke cases and subtype SVD cases than in controls. Moreover, the interplay between the DICER rs3742330 GG genotype and hyperlipidemia status was elevated stroke prevalence. The roles of DICER in angiogenesis and vascular growth have previously been investigated, and there is a reported association between DICER expression and healthy endothelial cell growth [39]. Moreover, there is strong evidence that a functional DICER1-dependent pathway is essential for a healthy endothelial angiogenic response. All major steps of the angiogenic process, including adhesion, proliferation, migration, and capillary-like structure formation are compromised by disrupted DICER1 signaling in cerebromicrovascular endothelial cells [40,41], in addition to other cell types [32,38,42,43].
DICER and DROSHA play crucial roles in vertebrate development. DICER1-deficient mice die early in development, between embryonic days 12.5 and 14.5, displaying impaired blood vessel and yolk sac formation. Similarly, zebrafish DICER mutant embryos display abnormal morphogenesis during gastrulation, brain formation, somatogenesis, and heart development [38]. In addition, loss of DROSHA leads to vascular smooth muscle cells disorder followed by hypoplastic blood vessel walls, cardiomyopathy, and liver hemorrhage in mice between embryonic days 13.5 and 14.5, and causes embryonic mortality in affected mice [44]. A number of studies have reported that DICER and DROSHA polymorphisms, including rs3742330 and rs10719, affect disease development and patient survival in various cancers [34,45-49]. In addition, functional analysis of rs1057035, which resides in the 3’-UTR of DICER, has revealed that the polymorphism affects hsa-miR-574-3p targeting and DICER expression [50]. The DROSHA rs10719 polymorphism, which is located in the 3’-UTR of DROSHA, was associated with different DROSHA expression levels [33] and presented different binding efficiency for the target site of hsa-miR-27b [35,51]. Furthermore, previous studies reported that DROSHA rs6877842 and rs640831 polymorphisms affected miRNA expression levels [34,52].
There are several potential mechanisms linking DICER and DROSHA polymorphisms to ischemic disease. First, DICER and DROSHA polymorphisms may directly affect angiogenesis via endothelial cell growth or induce blood vessel defects in embryos, resulting in vascular abnormalities [39,44]. In a DICER and DROSHA knockout model, hemorrhaging during vascular smooth muscle cell development was observed [39,44]. Moreover, DICER silencing in endothelial cells modulated the expression of several genes involved in endothelial biology, including nitric oxide synthase 3, matrix metalloproteinase 2 (MMP-2), integrins-v and -1, fibronectin, endothelin receptor types A, endothelin 1, vascular endothelial cadherin, and caspase-3. Both integrins-v and -1 are implicated in angiogenesis and endothelial survival [53], and MMP-2 participates in autocrine processes that influence hypoxia-induced migration and apoptotic death in endothelial cells [54]. Additionally, DROSHA has a similar role to DICER in vascular smooth muscle cell survival through ERK1/2 and AKT regulation [44]. Furthermore, previous studies identified that DROSHA influenced the regulation of miRNA expression. Transcription of certain miRNAs does not require DICER, but does need DROSHA (e.g., miR-1225 and miR-228) [55]. In addition, other miRNAs such as miR-877, miR-1224, and miR-1226 are independent of the canonical miRNA biogenesis pathway but dependent on the splicing process by DROSHA [56-58].
Alternately, DICER and DROSHA may indirectly affect miRNA regulation via RNA interference. As noted above, DICER and DROSHA are involved in miRNA biogenesis. miRNAs have multiple mRNA targets, are important regulators of gene expression, and play important roles in the initiation and progression of diverse diseases including leukemia, rheumatoid arthritis, and multiple sclerosis [59-62]. In particular, miRNAs are known to affect the immune system and vasculature in ischemic stroke [19,21,62]. At present, it is not known whether DICER polymorphisms affect stroke risk by affecting DICER enzyme function or via RNA interference. Further in vitro studies are needed to distinguish between these two hypotheses.
Interestingly, the results of the current study indicate a significant association between increased mortality after stroke and the RAN rs14035 C>T polymorphism, after adjusting for age, sex, hypertension, hyperlipidemia, and smoking status. As we did not have data on causes of death, we cannot be certain that the high mortality in patients with RAN rs14035 TT genotype was due to vascular events. However, analysis revealed that the RAN rs14035 TT genotype was significantly associated with the survival rate of ischemic stroke patients, supporting the likelihood of it also causing post-stroke mortality.
This was a case-control association study with 988 samples, but it has several limitations. First, although we found an association between XPO5 rs11077 polymorphisms and stroke risk, there is still no hypothesized mechanism for the role of this polymorphism in ischemic stroke prevalence. Second, the weak associations observed between the DICER rs13078, DROSHA rs6877842, and RAN rs14035 polymorphisms and ischemic stroke require replication. Third, some of the controls in our study were seeking medical attention; therefore, they were not completely healthy. However, recruitment of healthy participants with imaging and laboratory tests would markedly reduce the enrollment rate and including participants without imaging and laboratory tests may produce other vascular risk factor assessment biases. Finally, the study population was restricted to patients of Korean ethnicity.
Conclusions
We have identified an association between ischemic stroke susceptibility and polymorphisms in DICER rs3742330 and DROSHA rs10719, in addition to a significant association with the RAN rs14035 polymorphism in post-stroke mortality. These findings may encourage research efforts focusing on the role of DICER and DROSHA in vascular development. We postulate that the DICER rs3742330 and DROSHA rs10719 polymorphisms influence miRNA biosynthesis and therefore, miRNA post-transcriptional regulation during vascular endothelial cell growth, proliferation, and differentiation. However, the underlying mechanism remains to be elucidated in future research.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2017.02586.
PCR-RFLP condition for microRNA machinery genes polymorphism
Comparison of genotype frequencies and AOR of DICER 6095 rs13078 A>T, DICER 9480 rs3742330 T>C, DROSHA -715 rs6877842 C>G, DROSHA 4576 rs10719 T>C, RAN 1857 rs14035 C>T, and XPO5 4485 rs11077 A>C polymorphisms between stroke subtype and controls
AORs for ischemic stroke associated with microRNA machinery gene genotypes, stratified by clinical factors
Adjusted odds ratios for ischemic stroke associated with DICER genotypes, combined by clinical factors
Adjusted odds ratios for ischemic stroke associated with DROSHA genotypes, combined by clinical factors
Adjusted odds ratios for ischemic stroke associated with RAN and XPO5 genotypes, combined by clinical factors
Altered blood coagulation factors according to miRNA processing gene genotypes
Genotype frequencies of microRNA processing genes polymorphism and ischemic stroke patients’ mortality according to TOAST subgroup
Results of stepwise Cox regression analysis of ischemic stroke survival
Frequency of DICERA and DROSHA genotype combinations predicted by multidimensional reduction in ischemic stroke cases and controls
Allele combinations of DICER, DROSHA, RAN, and XPO5 polymorphisms between ischemic stroke patients and control subjects by multidimensional reduction method
Differences in activated partial thromboplastin time (aPTT) and antithrombin proportions based on DROSHA rs10719 T>C in ischemic stroke patients. Statistical analysis was performed using analysis of variance (ANOVA) test or Student t-test for each DROSHA rs10719 T>C genotype. (A) aPTT: the blood coagulation time was significantly different (P=0.005) between the DROSHA rs10719 TT (31.07±7.06), TC (31.20±7.30), and CC (36.23±35.28) genotypes. (B) Plasma antithrombin proportion: it was found that the DROSHA rs10719 T>C polymorphism affected the antithrombin proportion. The DROSHA rs10719CC genotype was associated with an elevated antithrombin percentage (97.32±27.29) compared with the DROSHA rs10719TT genotype (94.67±17.64), which had high antithrombin proportion relative to the DROSHA rs10719CC genotype (P=0.017). *P<0.05 calculated by ANOVA test; † P<0.05 calculated by Student t-test.
Notes
Disclosure
The authors have no financial conflicts of interest.
Acknowledgements
This study was partially supported by National Research Foundation of Korea Grants funded by the Korean Government (NRF2015R1D1A1A09057432, NRF-2016R1D1A1B03930141, and NRF-2017R1D1A1B03029582) and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI16C1559).