Hypercoagulability and Mortality of Patients with Stroke and Active Cancer: The OASIS-CANCER Study
Article information
Abstract
Background and Purpose
Patients with active cancer are at an increased risk for stroke. Hypercoagulability plays an important role in cancer-related stroke. We aimed to test whether 1) hypercoagulability is a predictor of survival, and 2) correction of the hypercoagulable state leads to better survival in patients with stroke and active cancer.
Methods
We recruited consecutive patients with acute ischemic stroke and active systemic cancer between January 2006 and July 2015. Hypercoagulability was assessed using plasma D-dimer levels before and after 7 days of anticoagulation treatment. The study outcomes included overall and 1-year survival. Plasma D-dimer levels before and after treatment were tested in univariate and multivariate Cox regression models. We controlled for systemic metastasis, stroke mechanism, age, stroke severity, primary cancer type, histology, and atrial fibrillation using the forward stepwise method.
Results
A total of 268 patients were included in the analysis. Patients with high (3rd–4th quartiles) pre-treatment plasma D-dimer levels showed decreased overall and 1-year survival (adjusted HR, 2.19 [95% CI, 1.46–3.31] and 2.70 [1.68–4.35], respectively). After anticoagulation treatment, post-treatment D-dimer level was significantly reduced and independently associated with poor 1-year survival (adjusted HR, 1.03 [95% CI, 1.01–1.05] per 1 μg/mL increase, P=0.015). The successful correction of hypercoagulability was a protective factor for 1-year survival (adjusted HR 0.26 [CI 0.10–0.68], P=0.006).
Conclusions
Hypercoagulability is associated with poor survival after stroke in patients with active cancer. Effective correction of hypercoagulability may play a protective role for survival in these patients.
Introduction
Systemic cancer is associated with an increased risk of ischemic stroke [1,2]. Active cancer increases the short-term risk of stroke, and ischemic stroke can be the first manifestation of systemic cancer [2,3]. Characteristics of stroke in patients with active cancer are distinct from those in patients without cancer; for example, embolic pattern and cryptogenic etiology are more frequently observed [1,4]. Cancer-related hypercoagulability seems to play an important role in the development of cancer-related stroke [5-7].
Overall survival after stroke is poor in patients with cancer [1]. Unlike conventional predictors for death in conventional stroke [8], cryptogenic mechanisms, lung cancer, and systemic metastasis are reported to be associated with poor survival in cancer-related stroke [1,9]. Each of those factors is linked to hypercoagulability [3,10]. If hypercoagulability is associated with increased mortality in cancer-related stroke, it is also of interest to investigate whether survival can be modified with effective antithrombotic treatment. Anticoagulation is associated with improved survival in patients with cancer and deep venous thrombosis [11-15]. However, there is a lack of evidence for the success of antithrombotic therapy in ischemic stroke. Furthermore, whether or not a successful correction of the hypercoagulable state can improve stroke survival has not been investigated.
We hypothesized that cancer-related hypercoagulability and its correction determines survival after stroke in patients with active cancer. We tested whether the level of pre-treatment plasma D-dimer, a surrogate marker of hypercoagulability, is associated with survival. In addition, we tested the plasma D-dimer levels after anticoagulation treatment to evaluate whether the successful correction of hypercoagulability can affect patient survival.
Methods
Patients
This is a part of the Optimal Anticoagulation Strategy In Stroke related to Cancer (OASIS-Cancer) study. The OASIS-Cancer study is an observational study to investigate the biological markers for intravascular coagulopathy causing stroke and for monitoring the effects of anticoagulation therapy in patients with active cancer and stroke (ClinicalTrials.gov identifier NCT02743052).
We recruited consecutive patients with acute ischemic stroke and active systemic cancer between January 2006 and May 2015 at the Samsung Medical Center, which is a university hospital with comprehensive stroke and cancer centers. During the study period, patients who 1) had acute ischemic stroke documented based on diffusion-weighted imaging within 7 days after symptom onset, 2) were admitted to or sought consultation at the neurology department for acute stroke, and 3) had known or newly diagnosed active systemic cancer at the time of stroke diagnosis or during hospitalization, were prospectively coded in our cancer-stroke registry. Active cancer was defined as a diagnosis of or treatment for cancer during the 6 months preceding the stroke diagnosis, or the presence of recurrent or metastatic cancer [7]. Local basal cell or squamous cell carcinoma of the skin and primary CNS tumors were not regarded as systemic cancers and were excluded. In case of newly diagnosed cancer, evaluations for tissue diagnosis and staging were performed by our oncologists. The institutional review board of the Samsung Medical Center approved this study. All participants provided written informed consent before participation.
Work-up for stroke
The age, sex, National Institutes of Health Stroke Scale (NIHSS) score, and stroke risk factors including hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, ischemic heart disease, and tobacco use were obtained for all the patients. The type of primary cancer, histology, and the presence of systemic metastasis were also recorded. Routine laboratory data were collected for all patients. Routine evaluations included electrocardiography, 24-hour Holter monitoring or 72-hour in-patient telemonitoring, echocardiography, brain magnetic resonance (MR) imaging, and MR angiography. Stroke mechanisms were determined using the Causative Classification System (CCS) in a weekly consensus meeting. Conventional stroke mechanisms included large artery atherosclerosis, cardioembolism, small-artery occlusion, and other evident mechanisms. In the absence of conventional stroke mechanisms, the mechanism was classified as cryptogenic.
Hypercoagulability measurements
Coagulation studies were performed as a routine protocol for acute stroke. Prothrombin time (PT) (s), activated partial thromboplastin time (s), fibrinogen (mg/dL), and D-dimer levels (µg/mL) were measured in patients with acute neurologic symptoms at their arrival in the emergency department. For in-hospital strokes, the coagulation studies were conducted immediately before the computed tomography (CT) testing. The D-dimer level was measured per protocol before antithrombotic treatment in all but five patients.
Antithrombotic treatment was determined based on the stroke mechanism. In patients with increased levels of coagulation markers without conventional stroke mechanisms, anticoagulation treatment was considered as the first-line antithrombotic treatment in the absence of obvious contraindications. Low-molecular-weight heparin (1 unit/kg twice a day) or adjusted-dose warfarin were used. During hospitalization, plasma D-dimer level was serially monitored after the start of the anticoagulation treatment to check whether it had normalized. In this study, the post-treatment D-dimer level measured at 7 (±3) days after anticoagulation was used to determine successful correction of hypercoagulability. The operational definition of 7 days was used because, after the first week, the continuation of antithrombotic agents and monitoring of D-dimer levels were highly influenced by patients’ clinical situations, including invasive procedures performed for tissue diagnosis, hematological abnormalities (e.g., thrombocytopenia), bleeding episodes, or refusal of further treatment. We set the cutoff level of post-treatment plasma D-dimer concentration at <3 µg/mL to define successful correction, which implies a shift from severe to non-severe hypercoagulability [16].
After the acute stroke setting, subjects who were regularly followed at our stroke outpatient clinic underwent serial plasma D-dimer level measurements at each outpatient visit. In this study, the outpatient plasma D-dimer levels were determined at 1-month (±15 days) intervals after onset.
Outcome
Survival status and death date were identified at June 1, 2016 by using data from the Korean National Health Insurance Service database. In Korea, medical insurance is provided to everyone under the National Health System [17]. In our cohort, information on the survival or death was complete in all except three patients who had foreign nationality. Overall survival was defined as the time from the stroke onset to the date of death as a result of any cause. To determine the overall survival of the surviving patients, survival time was censored at June 1, 2016. To avoid bias due to different follow-up periods, the length of survival was also censored at 1 year after stroke onset (1-year survival). Both overall and 1-year survival were investigated as the outcomes of this study.
Statistical analysis
The pre-treatment plasma D-dimer levels were divided into quartiles. Demographics, stroke characteristics, and cancer characteristics were compared among quartiles of pre-treatment D-dimer levels with the chi-square test, the Fisher’s exact test, or the Kruskal-Wallis test. Mortality rates, median survivals, and interquartile ranges were displayed using the Kaplan Meier method and survival curves were compared between the predefined prognostic factor groups with the log-rank test. A univariate Cox regression model was applied to each predictor of survival, while multivariate Cox regression was performed with a forward stepwise method using quartiles of plasma D-dimer concentrations, systemic metastasis, stroke mechanism, age, NIHSS score, primary cancer type, cancer histology (adenocarcinoma vs others), and atrial fibrillation. Among the primary cancer types, the most significant ones based on the log-rank test were selected as covariates in the multivariate model.
In patients with high (3rd and 4th quartiles) D-dimer levels who underwent anticoagulation therapy, the post-treatment D-dimer levels were tested using the Cox regression model for their overall and 1-year survival after stroke. Post-treatment plasma D-dimer levels were tested as both continuous and categorical variables (>3 vs. <3 µg/mL). In order to account for the possible influence of co-existing disseminated intravascular coagulopathy (DIC), subscores of the International Society on Thrombosis and Hemostasis (ISTH) scoring system [16], which consisted of platelet count (>100, 50–100, <50 x 103/µL), prothrombin time (<3, 3–6, >6 seconds), and fibrinogen level (>100 vs. <100 mg/dL), were adjusted for with a forward stepwise method as well as by using the aforementioned parameters as covariates in the model. An interaction term between pre-treatment and post-treatment D-dimer levels was added in the Cox regression model to test the post-treatment modification of the relationship between hypercoagulability and survival.
For each Cox regression model, proportional hazard assumptions were examined by testing Schoenfeld residuals for continuous variables and by visual examination of the log (minus log) curves for categorical variables. We used the variance inflation factor to assess the multicollinearity among variables in all the multivariate models. Results are reported as the hazard ratio (HR) with the 95% confidence interval (CI).
Plasma D-dimer levels collected in the outpatient clinic were tested for their association with mortality in the next month by using logistic regression analysis. The diagnostic value of D-dimer levels measured at any time in predicting death within the next month was determined with the receiver operating characteristic (ROC) curve. The Youden’s index (sensitivity+specificity–1) was used to determine the optimal cutoff for the prediction of death within the next month.
All statistical analyses were performed using commercially available software (SPSS for Windows, version 18.0; IBM Corp., Armonk, NY). A P value <0.05 was considered significant. Bonferroni correction was performed to correct for multiple comparisons, if any.
RESULTS
Patients
The study profile is shown in Figure 1. Of the 4,725 patients in the acute stroke registry during the study period, 271 (5.7%) were coded as having active cancer at the time of stroke onset. Among them, 268 patients had complete data on survival and death dates (Table 1). The median overall survival was 109 (interquartile range [IQR] 47–468) days. A total 224 of deaths were identified with a median (IQR) survival of 80 (36–209) days. The median (IQR) follow-up for 44 patients alive at the time of censoring was 2,302 (521–2,866) days. The mortality rate was 18.3% (47/268) at 1 month, 44.4% (117/268) at 3 months, 60.1% (159/268) at 6 months, and 71.6% (192/268) at 1 year.

Study profile. *Hypercoagulability and anticoagulation treatment were determined with a consideration of clinical situations. In this frequency analysis, >3 μg/mL was used as the cutoff of increased plasma D-dimer level.
Baseline characteristics
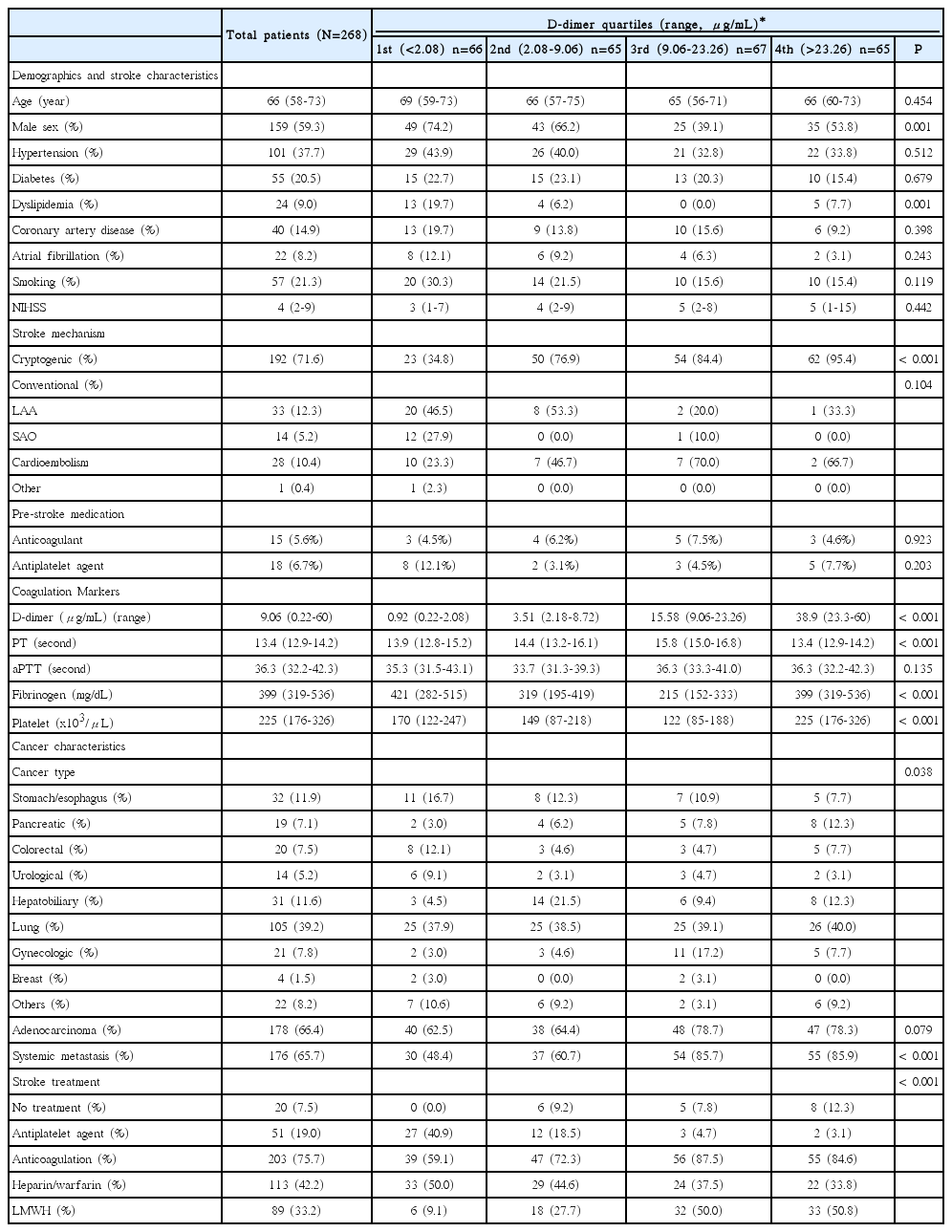
In 263 patients with D-dimer measurements obtained per protocol, the median pre-treatment plasma D-dimer level was 9.06 µg/mL (range 0.22–60.00 µg/mL). The quartiles of the pre-treatment D-dimer concentrations were determined: <2.08 µg/mL (1st quartile), 2.08–9.06 µg/mL (2nd quartile), 9.06-23.26 µg/mL (3rd quartile), and >23.26 µg/mL (4th quartile). Patient characteristics according to plasma D-dimer groups are described in Table 1. Patients with the lowest D-dimer levels (1st quartile) had more conventional stroke mechanisms and conventional risk factors such as male sex, dyslipidemia, and atrial fibrillation. In contrast, patients with higher D-dimer levels (3rd–4th quartiles) had more adenocarcinoma histology and systemic metastases, and subsequent anticoagulation therapy. Pancreatic and hepatobiliary cancers were less prevalent in patients with the lowest D-dimer levels (1st quartile). Pre-stroke anticoagulation treatment was not associated with plasma D-dimer quartiles (Table 1). Estimated 1-year mortality rates were 42.7%, 76.0%, 83.9%, and 86.3% in patients with 1st, 2nd, 3rd, and 4th D-dimer quartiles, respectively.
Hypercoagulability and other predictors of survival
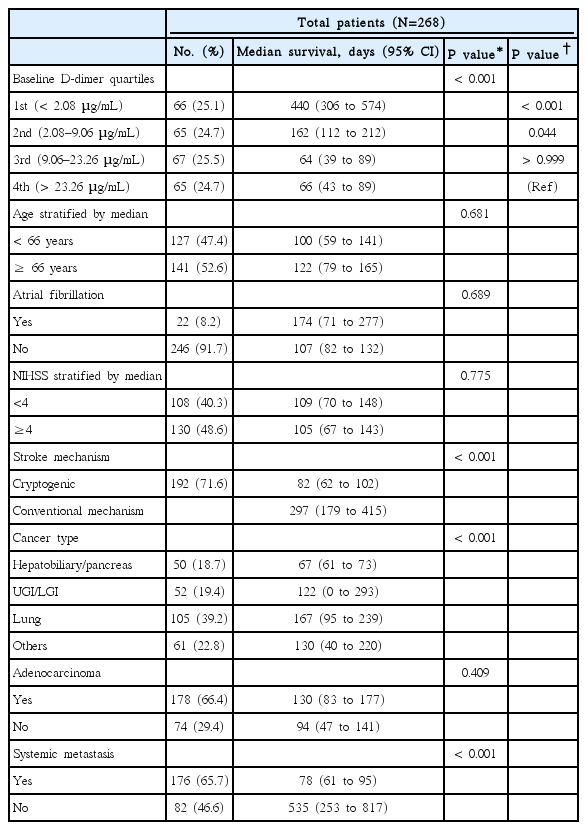
Kaplan-Meier analysis showed that higher baseline D-dimer quartiles, cryptogenic stroke mechanism, hepatobiliary-pancreatic cancer type, and systemic metastasis were significantly associated with poor survival (Log-rank test; all P<0.001, Table 2). Quartiles of baseline plasma D-dimer level provided significantly different survival curves, but were not different between 3rd and 4th quartiles for both overall and 1-year survival (both P>0.999 by log-rank tests after Bonferroni correction; Figure 2A, B). Therefore, we combined 3rd–4th quartiles together in the Cox regression model. Univariate analysis revealed that high (3rd–4th quartiles) pre-treatment plasma D-dimer levels were associated with increased mortality (HR 3.08, 95% CI 2.15–4.40 for overall survival; HR 3.78, 95% CI 2.48–5.75 for 1-year survival; Figure 2B). In the final multivariate model, patients with 3rd–4th D-dimer quartiles have more than two-fold higher hazard rate than those in the lowest quartile for overall and 1-year survival (adjusted HR, 2.19 [95% CI, 1.46–3.31] and 2.70 [95% CI, 1.68–4.35], both P<0.001; Figure 2C). The complete results of predefined predictors from Cox regression models are presented in the online Supplementary Table 1. The result remained significant in subgroups stratified by the presence of systemic metastasis and by stroke mechanisms (cryptogenic vs conventional mechanism) (see online Supplementary Table 2).
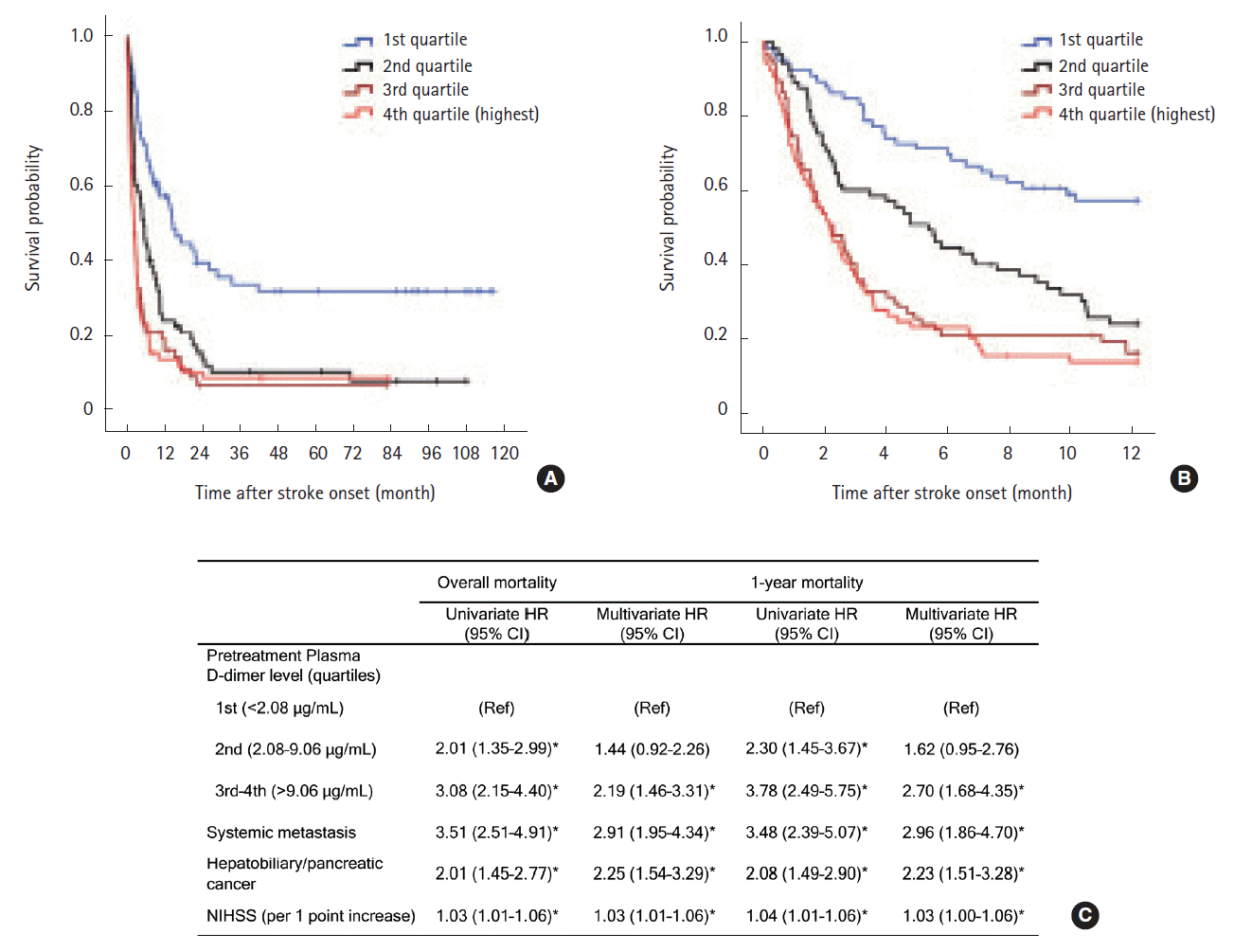
Kaplan-Meier survival curves by quartiles of pre-treatment plasma D-dimer levels (A) during the study period and (B) within 1 year after stroke (Log-rank test P<0.001 for both). (C) Univariate and multivariate Cox regression analysis for overall and 1-year survival after stroke. Age, systemic metastasis, NIHSS, primary cancer type (hepatobiliary-pancreatic vs others), atrial fibrillation, adenocarcinoma histology, and stroke mechanism (cryptogenic vs. conventional) were adjusted for in the multivariate model using a forward stepwise method. Only significant variables in each multivariate model are shown in the Table. *P<0.05.
Correction of coagulopathy and survival
Among the 132 patients with high D-dimer levels (3rd–4th quartiles), 113 (85.6%) underwent anticoagulation therapy. A minority of the patients (n=19) did not undergo anticoagulation because of bleeding diathesis (n=9) or severe stroke (n=10). In 113 patients who received anticoagulation treatment, the post-treatment D-dimer levels were sampled at a median of 7 (IQR 6–7) days after anticoagulation. Plasma D-dimer levels significantly reduced after treatment (mean difference: -16.22 µg/mL, 95% CI, -13.00– -19.43, P<0.001, paired t-test). The post-treatment D-dimer level was median 8.17 (IQR 3.70–14.21, range 0.46–55.18) µg/mL. In univariate Cox regression analysis, post-treatment D-dimer levels were significantly associated with both overall and 1-year survival (HR 1.02; 95% CI, 1.00–1.04 per 1 µg/mL increase, P=0.019 for overall survival; HR 1.03; 95% CI, 1.01–1.04 per 1 µg/mL increase, P=0.004 for 1-year survival). Multivariate analysis revealed that the post-treatment D-dimer level was independently associated with poor 1-year survival (adjusted HR 1.03; 95% CI, 1.01–1.05 per 1 µg/mL increase, P=0.015) but not with overall survival. Pre-treatment D-dimer level was no longer significant in association with survival in this subgroup. Rather, there was a significant interaction between pre-treatment and post-treatment D-dimer level on survival, whereby the impact of post-treatment D-dimer level on survival was moderated by the pre-treatment level with a negative coefficient (unstandardized beta=-0.002, SE=0.001, P for interaction=0.001 for both overall and 1-year survival).
Successful correction of hypercoagulability (<3 µg/mL) was achieved in 19 (16.8%) patients. Survival curves were clearly divided by the achievement of successful correction of hypercoagulability (log-rank P=0.011 for overall survival and 0.003 for 1-year survival; Figure 3A, B). In the multivariate Cox survival analysis, the successful correction of hypercoagulability was a protective factor for 1-year survival (adjusted HR 0.26 [CI 0.10–0.68], P=0.006; Figure 3C), not for overall survival. Hepatobiliary-pancreatic cancers and atrial fibrillation were also significant for 1-year survival after stroke (Figure 3C). Other covariates including baseline D-dimer concentrations, systemic metastasis, cryptogenic stroke mechanism, age, stroke severity, adenocarcinoma histology, platelet count, prothrombin time, and fibrinogen level were not significantly associated with survival. The baseline D-dimer concentrations and intervals of D-dimer sampling were not significant to the outcomes.
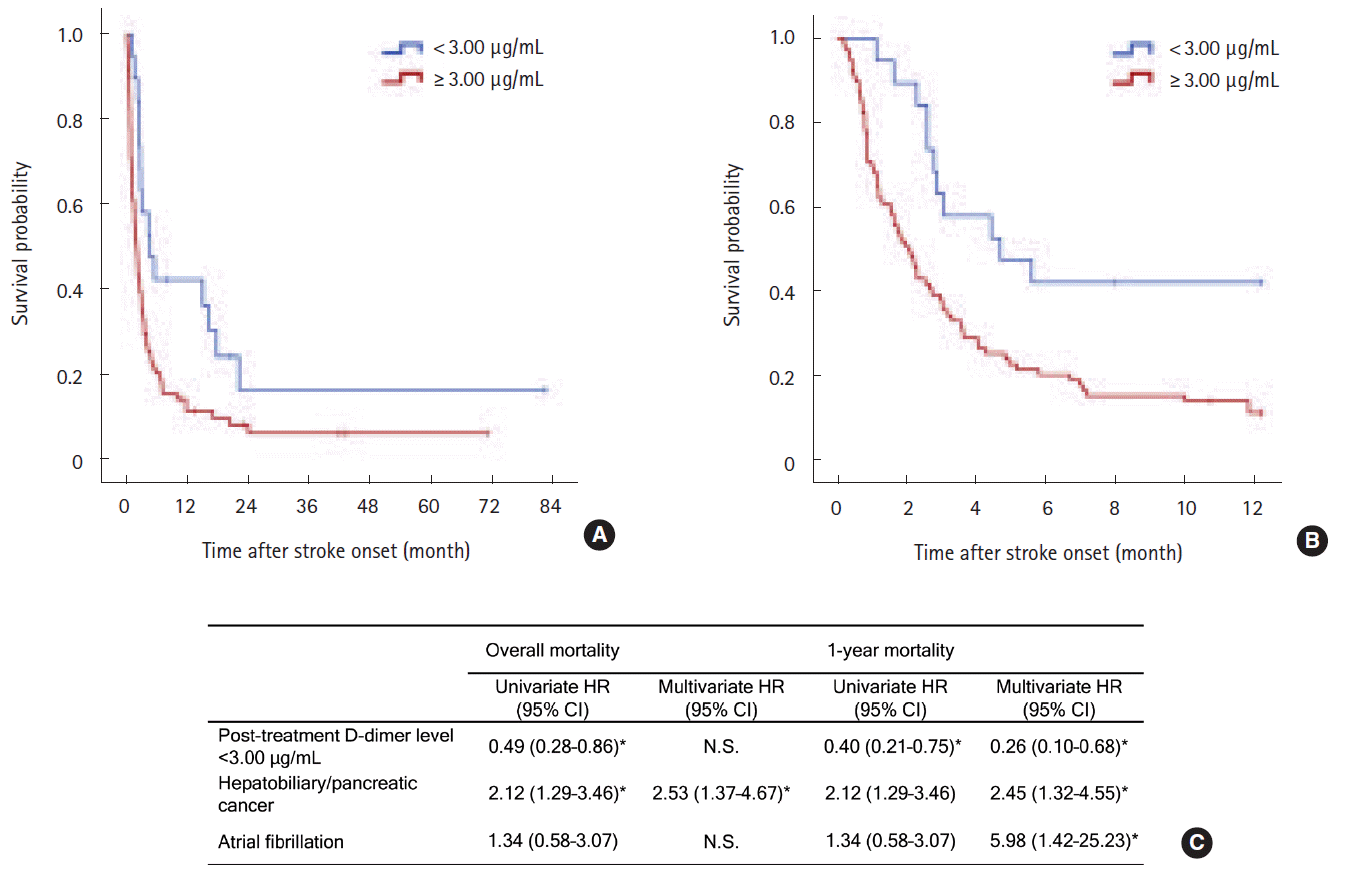
Kaplan-Meier survival curves (A) during the study period and (B) within 1 year after stroke by the achievement of successful correction of hypercoagulable states (D-dimer levels <3 μg/mL after anticoagulation) in patients with high baseline D-dimer levels (3rd and 4th quartiles) who underwent anticoagulation treatment (Log-rank P=0.011 and 0.003, respectively). Post-treatment plasma D-dimer levels were determined at 7 days after anticoagulation treatment. (C) Univariate and multivariate Cox regression analysis for overall and 1-year survival after stroke. Age, thrombocytopenia (>100, 50–100, <50 x 103/μL), hypofibrinogenaemia (>100 vs <100 mg/dL), prothrombin time (<3 vs >3 seconds), baseline plasma D-dimer concentrations, systemic metastasis, NIHSS, primary cancer type (hepatobiliary-pancreatic vs others), atrial fibrillation, adenocarcinoma histology, and stroke mechanism (cryptogenic vs conventional) were adjusted for in the multivariate model using a forward stepwise method. Only significant variables in each multivariate model are shown in the table. *P<0.05.
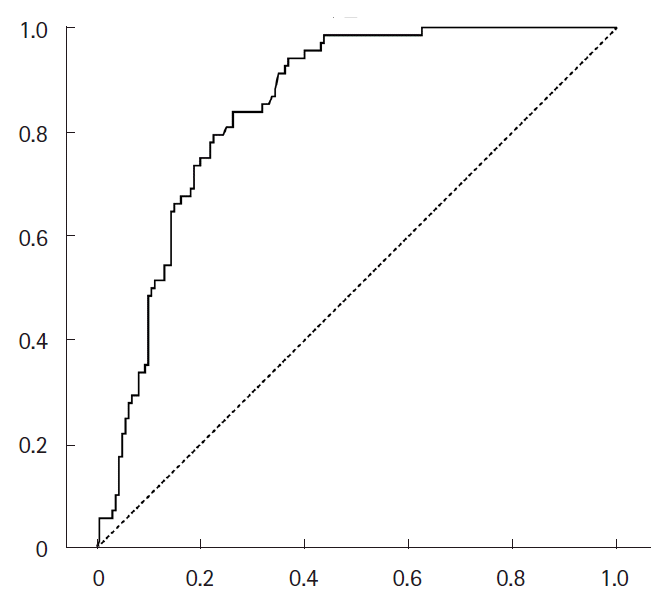
After discharge, plasma D-dimer levels were followed up at the outpatient neurology clinic in 136 patients. The plasma D-dimer level was significantly associated with death within 1 month (OR 1.07; 95% CI, 1.04–1.10 per 1 µg/mL increase, P<0.001). The ROC curve analysis revealed that the plasma D-dimer levels at any time was a significant predictor of death within the next month (area under the curve=0.835 [95% CI, 0.784 to 0.886], P<0.001) with an optimal cutoff of 3.17 µg/mL (Figure 4).
DISCUSSION
The major finding of the present study is that cancer-related hypercoagulability, as measured by plasma D-dimer levels, is associated with decreased survival in patients with stroke and active cancer. In patients with severe hypercoagulability, post-treatment plasma D-dimer level further divided survival curves within 1 year after stroke. Outside the acute stroke setting, higher plasma D-dimer levels were predictive of death within the next month of measurement.
Stroke in patients with cancer has distinctive features that differ from those of conventional stroke and also has a higher mortality [1]. In the present study, the mortality rate was 18.3% at 1 month and 71.6% at 1 year. Early mortality was driven mostly by patients with severe hypercoagulability, who had less conventional risk factors of stroke outcome. However, those patients also had more widespread metastasis and aggressive cancer types than patients without hypercoagulability. Therefore, it is both possible that hypercoagulability itself might be the leading cause of death, or a marker of more fatal nature of the cancer itself.
The association between cancer and increased thrombosis is well known. The tumor cell promotes a hypercoagulable state and activates the clotting cascade via tumor procoagulants such as tissue factors, cancer procoagulants, and tumor mucins [18]. Conversely, preclinical studies have shown that hypercoagulability can precipitate tumor growth or metastasis progression [18,19]. As a consequence of the bidirectional causal relationship, venous thromboembolism negatively affects survival in patients with cancer [20,21]. Similarly, hypercoagulability may play a role in survival after stroke in patients with cancer [6,7].
In this study, plasma D-dimer concentration, a surrogate marker of hypercoagulability, was an independent predictor of overall and 1-year survival after stroke in patients with cancer. Our study subjects had a high level of pre-treatment D-dimer levels with a median of 9.06 µg/mL, which is much higher than the levels observed in patients who had non-cancer strokes [22] and even large infarctions [23]. D-dimer, a stable end-product of fibrin degradation, is a direct measure of activated coagulation [1]. The plasma D-dimer level is a sensitive marker of cancer-associated hypercoagulable state [1,4,6,7,24]. We previously reported the significance of the plasma D-dimer level as a marker for diagnosis and treatment response in cancer-related stroke [3,7,25]. Extremely increased coagulation might also be associated with consumptive coagulopathy. Thus, it is unlikely that the association between mortality and high plasma D-dimer concentration is mediated by disseminated intravascular coagulopathy (DIC). In our multivariate model, post-treatment plasma D-dimer concentration was a significant predictor of death after controlling for DIC components [16,26]. In addition, our recent study results showed that the level of circulating cancer cell-derived extracellular vesicles [27], which are known to play a role in cancer progression (i.e., carcinogenesis/metastasis, coagulation, and tumor growth), correlated with plasma D-dimer levels [28].
The plasma D-dimer level after anticoagulation treatment was a significant predictor of 1-year survival in patients with severe hypercoagulability. Moreover, the interaction analysis showed that if the post-treatment D-dimer level is similar in all the patients, those who had higher pre-treatment D-dimer level, which implies marked reduction of D-dimer level after treatment, were more likely to survive. Conversely, those whose D-dimer level was relatively low but increased despite anticoagulation had worse survival outcome. Our finding suggests that the treatment response to anticoagulation is associated with better survival in patients with severe hypercoagulability. Evidence suggests that anticoagulants might prevent cancer progression and improve overall survival in patients with cancer [18,29]. In our study, in the subjects with high rate (85.6%) of anticoagulation treatment, anticoagulation itself could not be compared with other antithrombotic treatments. Rather, post-treatment D-dimer levels were followed up to assess whether hypercoagulability was effectively corrected. There might be several factors that influence the efficacy of anticoagulation, such as underlying cancer activity, coexistence with DIC, and intensity of anticoagulation treatment, of which the former two were controlled for in the multivariate analysis. Our study results encourage further prospective studies comparing fixed-dose anticoagulation and targeted therapy with careful monitoring of the coagulation status.
Our data imply that traditional prediction models of stroke mortality might not explain outcomes of patients with cancer-related stroke. Age has been used in various models of outcome prediction in patients with stroke [8,30,31], but was not significant in our study. On the other hand, cancer characteristics such as metastasis, primary cancer type, and cancer-related hypercoagulability had a considerable effect on survival in the present study. Contrary to previous reports [1], pancreatic and hepatobiliary cancers including cholangiocarcinoma were more unfavorable than lung cancers in our cohort. Hepatobiliary and pancreatic cancers may have an intrinsic fatality as they are often unresectable even in the absence of systemic metastasis [32-34]. Among lung cancers, adenocarcinomas were most common in our study subjects. Adenocarcinoma has been reported to be related to recurrent thromboembolism, which leads to poor survival in patients with cancer and stroke [35]. However, our findings suggest that both increased coagulation and primary cancer type determine the prognosis of adenocarcinomas. Atrial fibrillation was not an independent prognostic factor in the entire cancer-stroke cohort in our study, but led to poor 1-year survival in patients having severe hypercoagulability, possibly via an additive effect of slow flow and increased coagulation.
After the acute stroke setting, high plasma D-dimer concentrations measured in the outpatient clinic were associated with death within the next month. This could not be explained by stroke severity or consumptive coagulopathies. Instead, it is likely that increased coagulation can be a marker of increased cancer activity, effectiveness of antithrombotic treatment, or both. Although the causal inference could not be made in this observational study, data suggest that hypercoagulable states, if not effectively controlled, may lead to cancer progression via the action of thrombin on adhesion, mitosis, and angiogenesis in tumor cells [19]. Thus, monitoring of coagulation markers may be helpful even after recovery from stroke.
The strengths of this study include the large number of samples, a prospective collection of data, comprehensive and homogenous evaluations in a single tertiary center, long-term follow-up data during and after hospitalization, and high rates of survival identification. However, our study is not without limitations. First, cancer treatment was not included as a predictor of survival, because anticancer treatment is influenced by limited life expectancy, which can lead to a self-fulfilling bias [36]. Furthermore, both acute stroke and consequent functional impairment are often regarded as a contraindication of active anti-cancer treatment. A different strategy should be established to determine the optimal anti-cancer treatment in stroke survivors. Second, antithrombotic treatment was not controlled for in this observational study. However, most of the patients with severe hypercoagulability underwent anticoagulation treatment. We analyzed the post-treatment D-dimer levels only in subgroups that underwent anticoagulation treatment to minimize ascertainment bias. Third, co-existing deep venous thrombosis was not routinely evaluated during the entire study period. Venous thrombosis is a potential cause of increased D-dimer levels and ischemic stroke in the presence of right-to-left shunt [37]. However, our previous study demonstrated increased intra-arterial thrombosis and its association with increased D-dimer levels in cancer-related stroke [7]. We are conducting a prospective study to investigate the prevalence of deep venous thrombosis and right-toleft shunting in cancer-related stroke (clinicaltrials.gov identifier No. NCT02212496). Fourth, we did not determine the cause of death. Death in cancer patients can be attributed to cancer itself, as well as complications such as infection or thromboembolic events. Further research should be performed to reveal how increased coagulation affects survival. Finally, the data were evaluated from a single population. Our study subjects had a higher proportion of cryptogenic stroke than in previous studies [9]. This might be related, in part, to increased detection of hidden active cancer in cryptogenic stroke with hypercoagulable states in our center [3]. Further investigations of different study populations are needed before generalization of our results.
Conclusion
In conclusion, hypercoagulability is associated with poor survival in cancer-stroke, warranting monitoring and effective anticoagulation treatment for better survival. Plasma D-dimer levels might reflect the severity of the hypercoagulable states, treatment response, and risk of subsequent death in both acute hospitalized and chronic outpatient settings. Large-scale randomized prospective trials are needed to further evaluate the role of effective correction of hypercoagulability on survival after stroke in patients with active cancer.
Notes
This study was supported by the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1521).
The authors have no financial conflicts of interest.
Acknowledgements
The authors thank all the patients who participated in the study.
Supplementary Material
Univariate and multivariate Cox proportional hazard models for overall survival
Multivariate Cox proportional hazard models of overall survival in subgroups