Introduction
In the adult mammalian brain, there are at least two neurogenic regions: the ventricular-subventricular zone (V/SVZ) of the lateral ventricle and the subgranular zone of the dentate gyrus [1-5]. Neural stem cells within these two neurogenic niches generate new neurons throughout the life of the animal [6]. Within the adult rodent V/SVZ, the largest germinal niche, quiescent and activated neural stem cells coexist [7-9]. Activated neural stem cells divide to expand intermediate progenitor cells [7-9]. Neuroblasts generated by differentiation of the neural progenitor cells travel via the rostral migratory stream to the olfactory bulb, where they differentiate into granule and periglomerular neurons [1]. Adult neural stem cells in the V/SVZ also generate oligodendrocyte progenitor cells (OPCs) that disperse to the gray and white matter [10-13]. Focal cerebral ischemia in the adult rodent increases neurogenesis mainly in the V/SVZ, and augmented neuroblasts migrate from the V/SVZ to the ischemic boundary [2,14-16]. Stroke-induced neurogenesis has also been demonstrated in the adult human brain [17-19]. In addition, preclinical studies show that stroke also increases OPCs in the V/SVZ and these OPCs disperse to the peri-infarct region of the corpus callosum to differentiate into myelinating oligodendrocytes [20-24]. These findings, in particular neurogenesis, have led to a hope for re-establishment of damaged neuronal circuitry mediated by integration of stroke-induced new neurons [2,14-16]. However, subsequent experimental studies show that only a fraction of neuroblasts in the peri-infarct regions become mature neurons with phenotypes of interneurons, and these new neurons eventually die [25-27]. On the other hand, stroke continuously induces neuroblasts which migrate to peri-infarct regions for at least one year [28], and ablation of neuroblasts after stroke reduces ischemic brain repair and exacerbates functional recovery [29]. Together, these data suggest that stroke-increased neurogenesis is involved in the brain repair process via mechanisms that are independent of replacement of dead neurons to re-wire neuronal circuitry. In this review, we will detail the effect of stroke-induced neuroblasts and OPCs on brain parenchymal cells in ischemic brain with a focus on the brain repair processes.
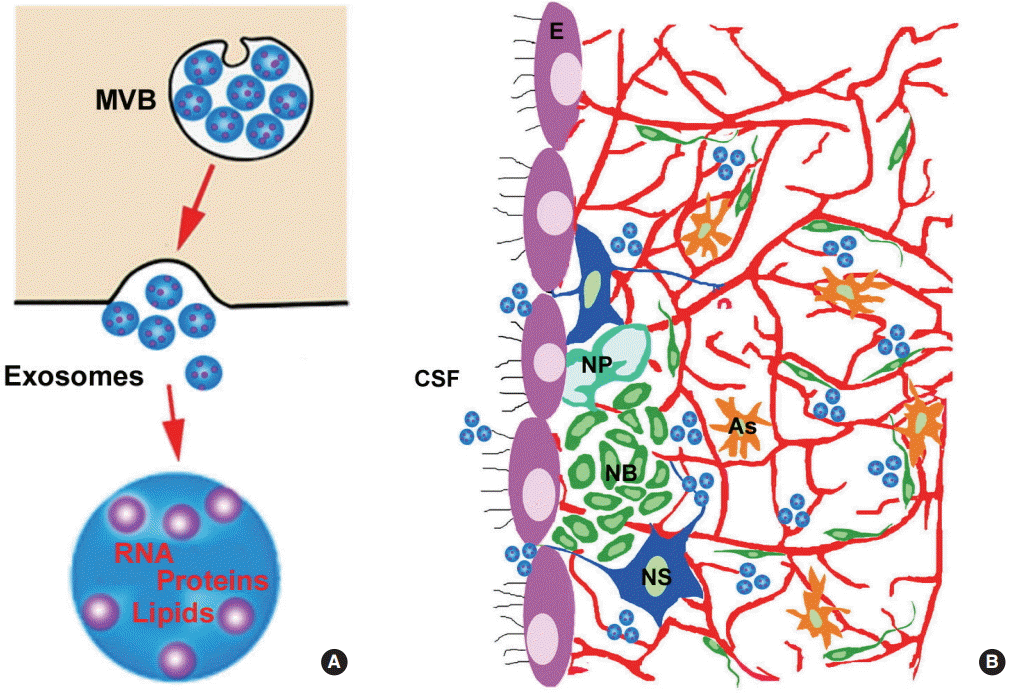
Coupling of neural stem cells, cerebrospinal fluid (CSF), and cerebral endothelial cells
In the adult V/SVZ neurogenic niche, cerebral blood vessels form a distinctive planar vascular plexus, and these blood vessels differ from the vascular structure in other brain regions, and permit small molecules to pass the blood brain barrier and to enter the SVZ [11,30,31]. Adult neural stem cells bridge the ventricle and the rich plexus of blood vessels in the SVZ via the apical single cilium of neural stem cells anchored on the ventricular surface and make direct contact with the CSF, whereas long basilar processes of neural stem cells eventually reach and directly contact blood vessels of this plexus in the SVZ [10,32]. Given this unique architecture, neural stem cells are well positioned to sample secreted factors in the CSF and to communicate with cerebral endothelial cells. Within the V/SVZ neurogenic niche, direct contact between neural stem cells and endothelial cells is essential to maintain adult neural stem quiescence [33]. Endothelial cells suppress active proliferation of neural stem cells via endothelial ephrinB2 and Jagged1 to interact with neural stem cell Notch and Eph, respectively [33]. Cerebral vasculature also releases factors, such as integrin α6 and β1, to regulate neural stem and progenitor cell biologic function [30,31]. Actively proliferating intermediate neural progenitor cells in the SVZ are localized to blood vessels [11]. These data indicate coupling of adult neural stem cells with cerebral endothelial cells [34]. Stroke robustly increases neural stem cells and new blood vessels within the V/SVZ niche [32]. Increased neural stem cell contact with the CSF and augmented blood vessels just beneath the ependymal layer suggest that quiescent adult neural stem cells in the V/SVZ niche may be recruited to an active pool to increase the neurogenic process in response to ischemic insult [32]. In vitro studies have demonstrated that primary cerebral endothelial cells isolated from ischemic brain promote proliferation and neuronal differentiation of non-ischemic SVZ neural progenitor cells, and neural progenitor cells isolated from ischemic SVZ enhance in vitro angiogenesis of non-ischemic cerebral endothelial cells [35]. The vascular endothelial growth factor/vascular endothelial growth factor receptor 2 signaling pathway mediates coupling of neural progenitor cells and cerebral endothelial cells [35]. In addition to the V/SVZ niche, increased neuroblasts induced by stroke in the V/SVZ migrate along cerebral blood vessels to peri-infarct regions where angiogenesis occurs [36-40]. Soluble molecules and their receptors mediate vascular-coupled neuroblast migration in ischemic brain, such as angiopoietin-1 (Ang1)/Tie2, stromal-derived factor-1α/chemokine receptor 4 (CXCR4), and among others [35-40]. Although new interneurons differentiated from neuroblasts in the peri-infarct regions eventually die, ablation of neuroblasts after stroke has an adverse effect on ischemic brain repair and worsens functional recovery [29]. These data suggest that increased neuroblasts are involved in brain repair processes after stroke.
Emerging data indicate that exosomes can also mediate the coupling of neural stem cells, CSF, and cerebral endothelial cells within the V/SVZ niche (Figure 1). Exosomes are endosome-derived nanovesicles and carry proteins, lipids, and genetic materials [41,42]. Exosomes play essential roles in intercellular communication by transferring their cargo between source and target cells under physiological and pathophysiological conditions [41,42]. There is evidence that exosomes in the CSF of rats and humans promote neural stem cell proliferation, possibly via delivering exosomal cargo protein and miRNA components of the insulin-like growth factor signaling pathway to neural stem cells [43]. Exosomes derived from ischemic cerebral endothelial cells facilitate proliferation and neuronal differentiation of adult neural progenitor cells, whereas exosomes harvested from ischemic neural progenitor cells increase in vitro angiogenesis [44]. These data suggest that exosomes mediate interactions between neural stem cells and cerebral endothelial cells under ischemic conditions. Thus, exosomes released by stroke-triggered neural stem cells and neuroblasts could communicate with brain parenchymal cells to amplify ischemic brain repair.
The effect of neural stem cells/neuroblasts on axonal remodeling
Neuronal circuitry regulates adult neural stem cell quiescence in the subgranular zone [45]. Axons from serotonergic (5HT) neurons in the raphe nuclei directly contact adult neural stem cells in the V/SVZ niche and regulate neural stem cell proliferation through the interaction of 5HT2 and 5HT2C receptor in neural stem cells [46]. However, it is unknown whether neural stem cells and neuroblasts have an impact on axons and neuronal circuitry. During cortical development, SVZ intermediate progenitor cells facilitate intracortical progression of thalamocortical axons through the stromal-derived factor-1α/CXCR4 signaling pathway [47]. Adult SVZ neural progenitor cells express stromal-derived factor-1α and CXCR4 [48]. In addition to the chemokine signals, adult neural stem cells in the V/SVZ release exosomes [44,49]. Cultured axons of cortical neurons can take up exosomes and miRNAs from internalized exosome cargo which thereby regulate axonal growth [50]. Stroke induces limited axonal sprouting in the peri-infarct region [21,22,51,52]. Extra-cellular factors and intrinsic signals of axons mediate axonal regeneration after axonal injury [53]. Thus, it will be important to determine whether the stromal-derived factor-1α/CXCR4 signaling pathway and exosomes released by neural stem cells in the V/SVZ and neuroblasts in periinfarct regions regulate axonal remodeling in ischemic brain.
The effect of neural stem cells on oligodendrogenesis
In the embryonic brain, OPCs arise from the VZ and migrate along vasculature throughout the brain [54]. Wnt and CXCR4 signaling mediate OPC-endothelial interactions to coordinate OPC migration and differentiation [54]. Adult neural stem cells in the V/SVZ also generate OPCs that comprise ~5% of the total cell number in the adult rodent brain and distribute throughout the grey and white matter [55-57]. OPCs are the most actively proliferating cells in the adult brain and differentiate into mature oligodendrocytes to myelinate previously unmyelinated axons [58]. Stroke acutely induces mature oligodendrocyte damage, leading to loss of myelin [59], which is associated with loss of axons [60,61]. New myelinating oligodendrocytes are generated by differentiation of OPCs. In contrast to neuroblasts, OPCs in ischemic brain survive and differentiate into myelinating oligodendrocytes [20-24]. As demonstrated by a cell fate mapping strategy, stroke increases neural stem cell-derived OPCs in the V/SVZ and promotes these OPCs to differentiate into myelin forming oligodendrocytes in peri-infarct white matter [20-24]. Thus, adult neural stem cells contribute to oligodendrogenesis after stroke. In addition to myelination, OPCs act as a surveillance network to detect brain injury and couple with cerebral endothelial cells [62,63]. Recent studies suggest that OPCs interact with microglia via OPC-released exosomes to regulate brain immune function [64]. Furthermore, OPC-exosomes promote neuronal survival under conditions of cell stress [65]. It remains to be investigated whether and how OPCs generated by adult neural stem cells are involved in brain repair processes other than oligodendrogenesis after stroke.
Conclusion
Stroke activates adult neural stem cell function in the V/SVZ, leading to neurogenesis and oligodendrogenesis in the ischemic brain. Although new neurons do not replace damaged neurons, stroke-increased neural stem cells and neuroblasts seem to participate in brain repair processes by communicating with cerebral vasculature and other brain parenchymal cells. OPCs generated by V/SVZ neural stem cells are also involved in ischemic brain repair processes. It will be important to investigate how neural stem cells, neuroblasts and OPCs communicate among themselves and with other brain cells, and in turn, mediate ischemic brain repair processes. These preclinical studies will potentially provide new strategies for enhancement of stroke-induced neurogenesis and oligodendrogenesis, consequently leading to improvement of neurological function after stroke. However, it remains to be demonstrated whether these inter-cellular communications between stroke-induced neurogenesis and parenchymal cells are involved in brain repair of patients with stroke.