Sleep Disturbances as a Risk Factor for Stroke
Article information
Abstract
Sleep, a vital process of human being, is carefully orchestrated by the brain and consists of cyclic transitions between rapid eye movement (REM) and non-REM (NREM) sleep. Autonomic tranquility during NREM sleep is characterized by vagal dominance and stable breathing, providing an opportunity for the cardiovascular-neural axis to restore homeostasis, in response to use, distress or fatigue inflicted during wakefulness. Abrupt irregular swings in sympathovagal balance during REM sleep act as phasic loads on the resting cardiovascular system. Any causes of sleep curtailment or fragmentation such as sleep restriction, sleep apnea, insomnia, periodic limb movements during sleep, and shift work, not only impair cardiovascular restoration but also impose a stress on the cardiovascular system. Sleep disturbances have been reported to play a role in the development of stroke and other cardiovascular disorders. This review aims to provide updated information on the role of abnormal sleep in the development of stroke, to discuss the implications of recent research findings, and to help both stroke clinicians and researchers understand the importance of identification and management of sleep pathology for stroke prevention and care.
Introduction
Stroke incidence and early stroke mortality have been decreasing, at least in developed countries, although stroke remains one of leading causes of death and significant disability worldwide [1-5]. Identification and active modification of risk factors, as well as progress in acute stroke care, underlie the improvements in stroke statistics. Modifiable risk factors such as high blood pressure, hyperlipidemia, diabetes, smoking, physical inactivity, and unhealthy diet are responsible for 90% of the risk of stroke [6,7]. However stroke incidence has not dropped significantly in young adults, and is still soaring in low and middle income countries [2,3,8]. The absolute numbers of stroke victims and resulting deaths, and the associated societal burden are great and even increasing [2]. Insufficient modification of the established risk factors, or the ongoing effects of under-recognized risks, might explain the high global burden of stroke. Recently, the role of sleep pathology in the development of cardiovascular and metabolic diseases has been highlighted by experimental and observational studies [9-15].
Sleep is an indispensable part of life, as with feeding and reproduction, all animal species require sleep. Humans sleep almost one-thirds of their lifetime, which is similar in industrialized and in pre-industrial societies [16,17]. Sleep, although characterized by quiescence and diminished responsiveness, is not a simple state of rest, but rather a cyclic state of periodic transitions between rapid-eye-movement (REM) and non-REM (NREM) sleep, which are precisely regulated by the central nervous system [18]. Along with the brain and other organs or physiological streams, the cardiovascular system achieves homeostatic restoration during sleep, mainly through autonomic circulatory control [19]. For example, the decrease in blood pressure during sleep, “dipping,” is a key biomarker of cardiovascular health, secondary to changes in activity and posture and also under the influence of sleep and circadian rhythms [20,21]. During NREM sleep, the largest portion (up to 80%) of normal adult sleep, the autonomic system is stabilized with vagal dominance, reduced sympathetic tone, and heightened baroreceptor gain, contributing to a significant reduction in blood pressure and heart rate, with the greatest drop occurring during slow wave sleep [21-24]. This salutatory milieu makes it easy for the cardiovascular system to maintain homeostasis. In contrast, REM sleep—occupying about 20% of total sleep—is dominated by marked fluctuations in sympathovagal balance (irregularly peaking sympathetic surges against a background of tonic vagal inhibition), which lead to abrupt changes in blood pressure and heart rate [9,19,22,24]. A compromised cardiovascular system is at risk for pathological events such as myocardial ischemia or arrhythmias during REM sleep. Sleep thus acts as a gatekeeper through cyclic oscillations between NREM and REM sleep, switching between autonomic tranquility and turmoil.
Any causes of sleep curtailment, including sleep restriction, insomnia, and shift work, are likely to impair cardiovascular restoration through a reduction in cardioprotective stable NREM sleep. Sleep fragmentation, conventionally defined by cortical EEG arousals, is a universal feature of almost all sleep disorders, including sleep apnea, insomnia, periodic limb movements during sleep (PLMS) and narcolepsy, and is associated with overshoots in sympathetic activity [25,26]. Even if REM sleep is preserved, the greater the sleep fragmentation, the farther the sympathovagal modulation is tilted toward sympathetic dominance [27]. Blood pressure regulation during the biological night (the usual sleep time) is uniquely related to cardiovascular risk, including the risk of stroke [28-30]. Non-dipping—loss of the typical blood pressure drop during sleep—is associated with a host of poor cardiac, neurological, metabolic, and renal outcomes [31-36]. Sleep fragmentation causes non-dipping [37-40]. Non-dipping is common in older adults and is associated with an increased risk of stroke [41-43]. Reduced dipping is associated with brain atrophy, worse functional status, and lower daytime cerebral blood flow [44,45].
The deleterious effects of sleep disturbances go far beyond autonomic circulatory derangement. Common sleep disorders such as sleep apnea, insomnia, and PLMS activate multiple mechanisms including intermittent hypoxia-reoxygenation injury, inflammation, insulin resistance, hypothalamic-pituitary-adrenal axis activation, hemodynamic swings, cardiac arrhythmia, and hypercoagulability, all of which have the potential to provoke cardiovascular diseases (Figure 1) [9,46].
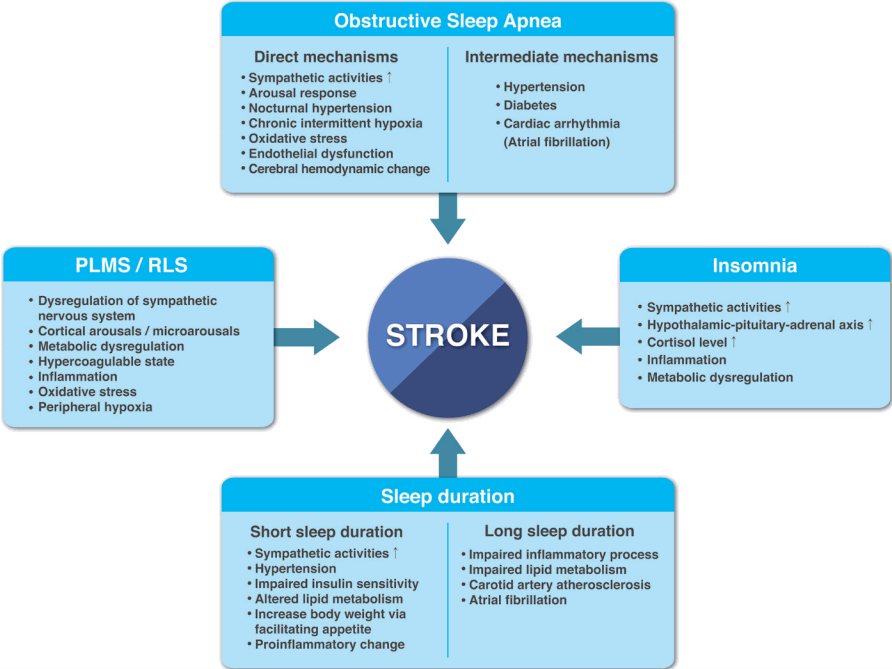
The mechanisms of sleep disturbances contributing to the development of stroke. PLMS, periodic limb movements during sleep; RLS, restless legs syndrome.
However, compared with studies on well-established risk factors, research on the role of sleep pathology in the development of cardiovascular diseases, including stroke, has largely been observational or experimental [13,47-58]. To complicate matters, recent randomized trials have failed to determine the beneficial effect of positive airway pressure therapy in the secondary prevention of cardiovascular events or mortality in sleep apnea [59-61]. However, serious flaws are pervasive in these studies, including poor use of therapy. This review aims to (1) provide an update of the available information regarding the role of sleep disturbances on the development of stroke; (2) discuss the implications of recent clinical trials and observational studies; and (3) help both stroke clinicians and researchers understand the importance of identification and management of sleep pathology for primary prevention in the general and stroke-prone populations, and for the care of acute and chronic stroke.
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is characterized by repetitive cessations or reductions in airflow caused by complete or partial upper airway collapse (Figure 2). The prevalence of moderate-to-severe OSA in the adult general population is 9% to 14% in men and 4% to 7% in women [62-65], increasing with age [66], and even higher in a recent study (49.7% in men, 23.4% in women) [67]. Measurement methods and scoring criteria heavily impact reported prevalence rates. Nevertheless, the prevalence and incidence of OSA is clearly increasing, driven by the aging of societies and the obesity epidemic [68]. Four decades after a report on the high prevalence of hypertension in pediatric and adult OSA [69], numerous experimental and observational studies have provided evidence that OSA promotes the development of cardiovascular diseases, including stroke [9,12,46,70].

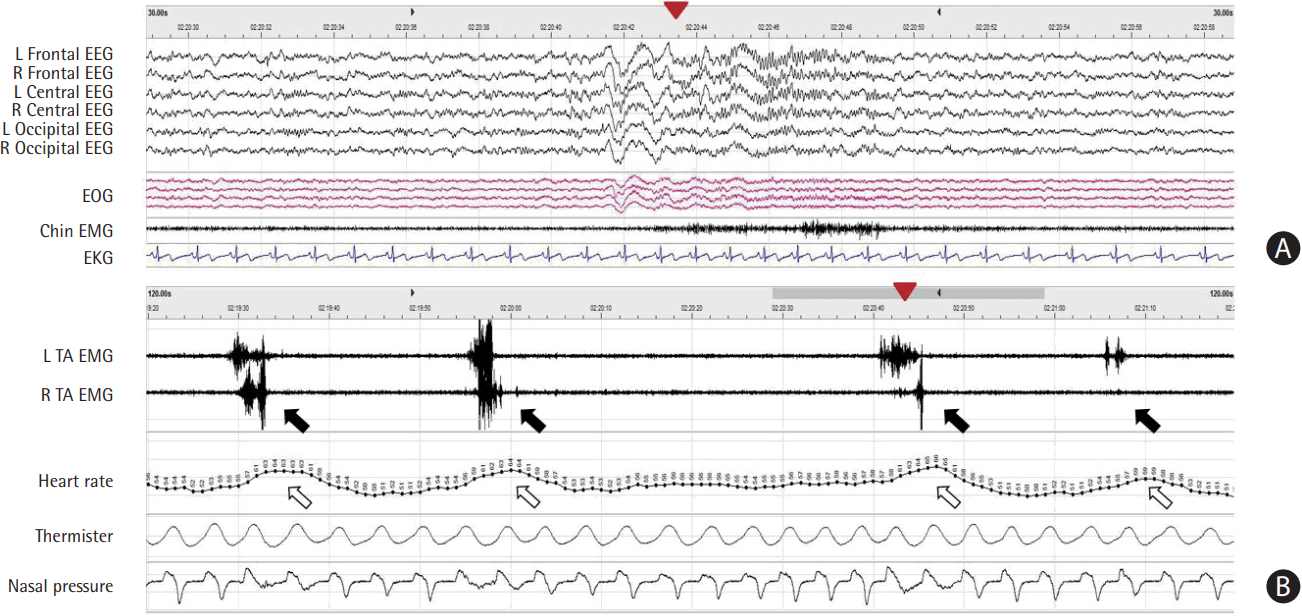
Snapshot of recurrent respiratory events in obstructive sleep apnea. (A) Electroencephalography (EEG) plots for 30-second epoch. Respiratory arousal (closed arrowhead in panel A) occurs at the end of obstructive sleep apnea (closed arrowhead in panel B) in stage 2 rapid-eye-movement (REM) and non-REM sleep. The closed arrowheads on panel A and B indicate the same time. (B) Respiratory plots for three-minute epoch. Repetitive cessations of airflow (closed arrows) despite ongoing respiratory movements of the chest and abdominal belt are typical features. Obstructive sleep apnea is accompanied by decreased oxygen saturation (open arrows) and increased heart rate (open arrowheads). L, left; R, right; EOG, electrooculography; EMG, electromyography; EKG, electrocardiogram; SpO2, peripheral oxygen saturation.
Links between OSA and stroke
OSA creates a substrate for stroke vulnerability, and is particularly hostile to brain function. Exposure to intermittent hypoxia in rodents results in impaired executive function, excessive sleepiness, and sensitivity to sleep deprivation [71-74]. There is evidence of neuronal injury in the hippocampus [75,76], the basal forebrain [77], and the wake-promoting catecholaminergic system [78]. The mediating mechanisms include free radical injury, lipid peroxidation, induction of nitric oxide synthase, platelet activation, and apoptosis [79-81]. However, oxidative stress, especially modest hypoxia, may have a protective effect on the brain and cardiovascular system by activating gene programs that induce vascular remodeling as well as other protective responses; thereby, engendering resilience in the brain (known as ischemic preconditioning) [82].
OSA is related to the surrogate index of stroke. Moderate-tosevere OSA is associated with silent ischemic changes, including white matter changes and lacunae as well as cerebral microbleeds [83-86]. Carotid and intracranial atherosclerosis are also accelerated in OSA [87,88]. It is unclear whether continuous positive airway pressure (CPAP) has a therapeutic effect on these changes [89].
Hypertension and insulin resistance might mediate the development of stroke in OSA. Moderate-to-severe OSA is significantly associated with prevalent and incident hypertension in a severity-dependent manner [90,91], and is highly prevalent in patients with resistant hypertension [92]. Effective CPAP therapy, alone or in addition to antihypertensive medication, significantly reduces blood pressure [93-95]. OSA may also increase the risk for development of type 2 diabetes by mechanisms such as increased insulin resistance and high cortisol secretion [12,96]. In a 6-month parallel trial, the CPAP group achieved a greater decrease in HbA1c (–0.4%; 95% confidence interval [CI], –0.7% to –0.04%) than the control group [97]. Continuously supervised CPAP therapy (7.92 hr/night) improved glycemic control and insulin resistance [98]. However, the CPAP effect on glycemic control is less consistent than its effect on blood pressure. Concomitant obesity might have a stronger effect than OSA, not mitigated by CPAP therapy.
OSA is also associated with the risk for cardioembolism. Nearly 40% of symptomatic atrial fibrillation events are seen between midnight and 8:00 AM [99]. People with OSA have four times the odds of atrial fibrillation (odds ratio [OR], 4.02; 95% CI, 1.03 to 15.74) [100]. Nocturnal oxygen desaturation is an independent risk factor for new onset atrial fibrillation [101]. In a recent cohort study of 6,841 patients, OSA diagnosis and severity were associated with atrial fibrillation (hazard ratio [HR], 1.55; 95% CI, 1.21 to 2.00) during a follow-up of 12 years [102]. Furthermore, OSA may potentiate the risk of cardioembolism or stroke in patients with atrial fibrillation [103,104]. Several observational studies found an improvement or resolution of cardiac arrhythmia and atrial fibrillation after CPAP therapy [105,106].
In addition, sleep apnea is associated with inflammation [107-110], endothelial dysfunction [111,112], hypercoagulability [113-115], and cerebral hemodynamic changes [116-118]. The overall findings suggest that OSA contributes to the development of stroke through various mechanisms downstream to intermittent hypoxia, sleep fragmentation, and hemodynamic swings [9,12,46,70].
Observational findings
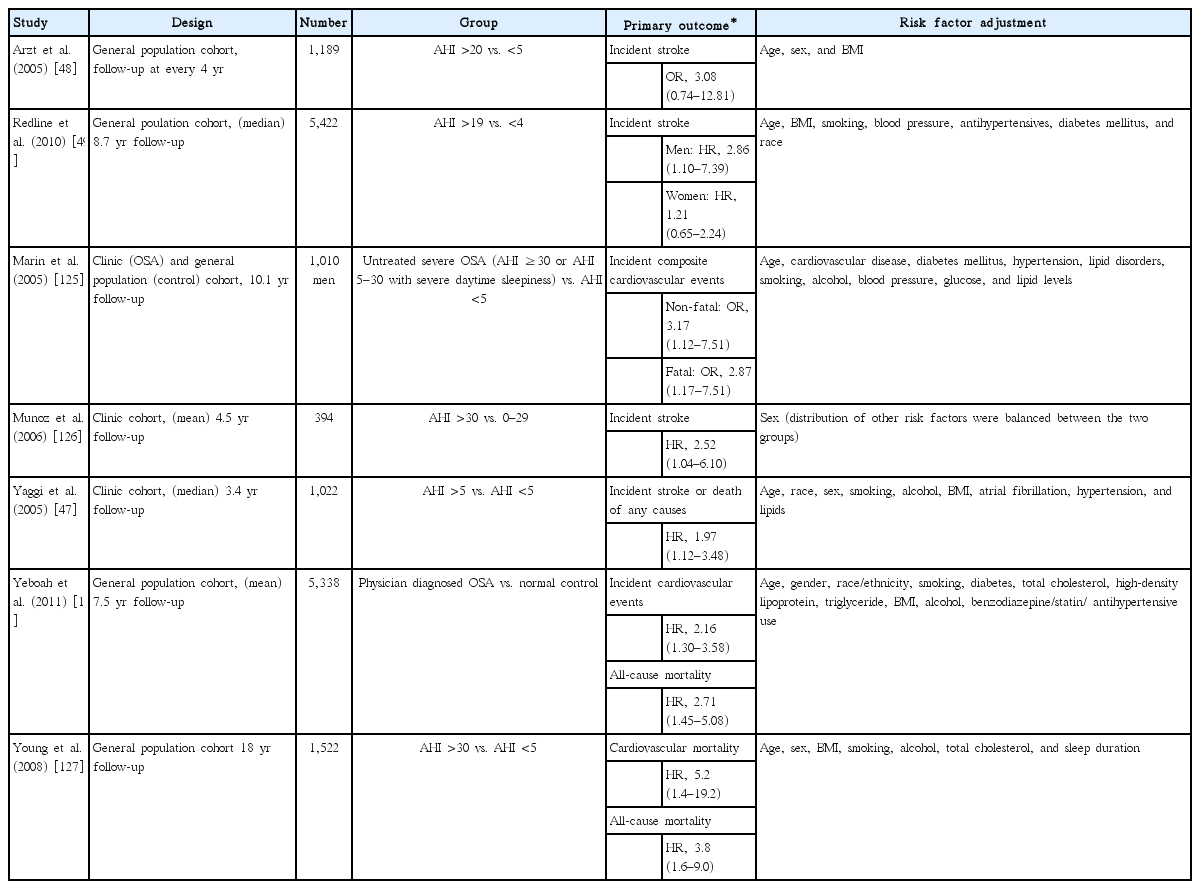
Sleep apnea is exceedingly common in the acute stroke setting, present in 50% to 70% of subjects with acute stroke or transient ischemic attacks, which is a higher frequency than observed in control groups [119-122]. However, OSA and stroke share risk factors such as male sex, obesity, old age, hypertension, and smoking [62,65,66,68]. An independent association between the two conditions was supported by large scale epidemiological studies including the Sleep Heart Health Study and the Wisconsin Sleep Cohort Study [48,123]. In these studies, OSA with an apnea-hypopnea index (AHI) ≥20/hr or >11/hr was related to prevalent stroke, with an OR of 4.31 (95% CI, 1.31 to 14.15) and 1.58 (95% CI, 1.02 to 2.46), respectively, when adjusted for age, sex, weight, blood pressure, smoking, and other confounders. Prospective cohort studies in clinical or general populations followed, and the major findings are summarized in Table 1 [47-49,124-128].
In the first study on the temporal relationship between OSA and stroke in the general population (from the Wisconsin Sleep Cohort), the causal association was attenuated (OR, 3.08; 95% CI, 0.74 to 12.81) when adjusted for age, sex, and body mass index [48]. The younger age of the cohort (mean age, 47±8 years old) and a low incidence of stroke (1.3 per 1,000 person-years) probably weakened the statistical power. In the Sleep Heart Health Study (n=5,422; stroke incidence, 4.4 per 1,000 person-years), men with moderate-to-severe OSA (AHI >19/hr), but not women, had a higher risk for ischemic stroke during follow-up (median, 8.7 years; HR, 2.86; 95% CI, 1.1 to 7.4). The lack of association in women was explained by insufficient statistical power (lower stroke incidence in women); the relatively shorter duration of OSA exposure (due to the later, usually postmenopausal, onset in women) [129]; the higher rate of OSA progression along with aging during the follow-up, leading to a considerable probability of misclassification (OSA vs. control, or OSA severity); and the stronger effects of well-known vascular risk factors [49]. For elderly people of both sexes (aged 70 to 100 years, n=394), the risk of incident stroke was significantly higher for severe OSA (AHI ≥30/hr; HR, 2.52) throughout a 6-year period of observation, independent of other vascular risk factors [126].
Prospective observational studies of clinic-based cohorts, assessing composite cardiovascular events as a primary endpoint, also suggested a role for OSA in the development of stroke [124,125]. Peker et al. [124] reported an independent association of OSA with cardiovascular events in a small community sample of men (age, 30 to 69 years old; n=182) free from prevalent cardiovascular diseases, hypertension, and diabetes. During the 7-year follow-up, the OR for people with OSA having cardiovascular diseases (including stroke) was 4.9 (95% CI, 1.8 to 13.6), and effective treatment of OSA significantly reduced the risk (OR, 0.1; 95% CI, 0.0 to 0.7). The effect of treatment, as well as the contribution of OSA to cardiovascular event incidence, were demonstrated in a subsequent long-term observational study of a clinical population (mean observation period, 10.1 years; n=1,656) [125]. The group of subjects with untreated severe OSA (AHI ≥30/hr or 5 to 29/hr with severe sleepiness) had a higher hazard for fatal and non-fatal cardiovascular diseases (HR, 2.87 and 3.17, respectively), compared with age- and body mass-matched controls. With an average nightly use of CPAP for at least 4 hours, the risk of incident cardiovascular events among subjects with severe or symptomatic OSA was comparable to that of controls or simple snorers (AHI <5/hr). The cardiovascular effects of OSA are not limited to subjects with a severe burden of OSA. A prospective observational study in a clinical population (aged ≥50 years; n=1,022) free from myocardial infarction or stroke at study entry, found that the presence of OSA (AHI ≥5/hr) increased the risks for outcome events (stroke or death from any cause, HR, 1.97; 95% CI, 1.12 to 3.48) during the follow-up (median, 3.4 years) [47]. However, the treatment effect was not substantial, even though a large proportion of OSA subjects had active treatments including meaningful weight reduction (31% with weight loss of ≥10% body weight), CPAP therapy (58%) with fair adherence (at least 4 hours of nightly use for ≥5 nights a week), and upper airway surgery (15%). The increased rates of stroke and death despite OSA treatment can be explained by older age (compared to a previous study [125]), with correspondingly increased vascular risk, prolonged exposure to OSA prior to the treatment, a relatively shorter duration of intervention, and changes in treatment effectiveness (weight regained, reduced CPAP adherence, or loss of surgery effect) [47]. The study was not designed a priori to investigate treatment effects. An increased risk of cardiovascular and non-cardiovascular death in the OSA group was also reported from a longitudinal analysis of the Wisconsin Sleep Cohort and the Multi-Ethnic Study of Atherosclerosis cohort [127,128].
Summarizing the observational findings, prospective studies largely support a causal relationship between OSA and stroke (Table 1). Recent systematic meta-analyses reported that OSA was significantly associated with incident stroke (OR, 2.24; 95% CI, 1.57 to 3.19) (relative risk [RR], 2.02; 95% CI, 1.40 to 2.10) [50,130]. However a few important issues should be considered. First, the effects of well-established cardiovascular risk factors other than age, including hypertension, diabetes, atrial fibrillation, and smoking, were smaller than that of OSA, which was generally not significant [47,49,125,126]. Optimal modification or treatment of well-established vascular risk factors was likely to reduce their influences on the outcomes in the study participants [126]. Second, the observational findings are inherently limited by study design and lack of randomization. The baseline characteristics among groups may differ by undefined factors other than chance. The “healthy adherer” effect, describing a better outcome in subjects compliant with any given intervention due to their health-conscious behavior or lifestyle, might bias the results in favor of CPAP therapy [131,132]. Studies with randomized designs are required to provide a better understanding of the causal relationship between OSA and stroke, as well as the therapeutic efficacy of CPAP in the prevention of stroke.
Randomized clinical trials
Well-designed randomized clinical trials (RCTs) of CPAP therapy for primary prevention of clinical outcomes such as stroke, coronary heart disease, or atrial fibrillation are scarce [133]. In an RCT, feasibility and study design are critically influenced by the expected rate of the primary endpoint, the predicted efficacy (and precision in application) of therapy, and the estimated duration of intervention, all of which affect the sample size required for sufficient statistical power. To examine the effects of interventions on hard clinical endpoints (e.g., stroke in OSA) requires a larger sample size and longer period of observation than needed for trials with surrogate endpoints (e.g., blood pressure or inflammation in OSA). Therefore, to assess the effect of therapy (e.g., CPAP) on clinical outcomes, secondary prevention studies may be more feasible [134,135].
Recently the results from two RCTs were reported, testing the long-term efficacy of CPAP therapy for the prevention of cardiovascular events in subjects with established cardiovascular disease (coronary heart disease or stroke) and OSA [60,61]. The Sleep Apnea Cardiovascular Endpoint (SAVE) trial was a multinational RCT including 2,717 non-sleepy subjects with moderate-to-severe OSA (AHI ≥15/hr, but not with severe hypoxemia [oxygen desaturation <80% for >10% of recording time]) and established stroke or cardiovascular disease, who were subsequently randomized either to usual care with CPAP therapy or to usual care alone, and were followed for a mean period of 3.7 years [60]. The study tested the hypothesis that additional CPAP therapy reduced the risk of future cardiovascular events (a composite of vascular mortality, myocardial infarction, stroke, or hospitalization for vascular events). During the follow-up, the CPAP therapy did not effectively lower the incidence of predefined events compared with the usual care (HR, 1.10; 95% CI, 0.91 to 1.32). In the CPAP group (n=1,346), the adherence was only 3.3 hours per night on average, and fair (≥4 hr/night) in less than half of the subjects (42%). During the run-in period of a week with sham CPAP that was introduced to maximize the treatment adherence of the study participants, the required threshold of minimal adherence was an average of 3 hours per night, equivalent to 50% adherence in those who sleep 6 hours daily. In the prespecified propensity-score-matched analysis to compare the group with fair CPAP adherence (n=561) with matched controls, the reduction in the composite endpoint was not significant (HR, 0.80; 95% CI, 0.60 to 1.07), but a lower risk of stroke was found (HR, 0.56; 95% CI, 0.32 to 1.00; P=0.05). Another RCT, the Randomized Intervention with CPAP in CAD and OSA (RICCADSA) trial, was performed in patients with coronary artery disease and concomitant non-sleepy OSA (n=244; AHI ≥15/hr) [61]. During the follow-up (median, 56.9 months), the incidence of the primary composite endpoint (repeat revascularization, myocardial infarction, stroke, or cardiovascular mortality) was not different between the groups with and without CPAP therapy, but based on the per protocol analysis, the risk was significantly lower in the CPAP adherent group (nightly use ≥4 hours) than in the non-compliant group or the untreated group (HR, 0.29; 95% CI, 0.10 to 0.86).
Both the large (SAVE) and the small scale (RICCADSA) RCTs failed to demonstrate efficacy of CPAP therapy. Negative findings in such high-profile trials underscore the need for future trials, and are vital contributors to recognizing and addressing the key factors to be considered in the design of future studies [136,137]. Several important aspects should be discussed in this context. Both trials included only non-sleepy OSA for ethical reasons (the expected harm to sleepy subjects if included but untreated). Excessive daytime sleepiness is a marker of higher levels of inflammation, insulin resistance, and blood pressure, compared with non-sleepy OSA [138-140]. By excluding sleepy subjects, the study sample was limited to a potentially lower-risk group, thus reducing its statistical power. Preceding RCTs on non-sleepy OSA populations have demonstrated that CPAP therapy is not effective in reducing blood pressure and preventing cardiovascular risk or events including incident hypertension, despite improvements in subjective sleepiness [141-145]. Therefore the lack of CPAP efficacy in trials performed with non-sleepy subjects cannot be generalized to severe or symptomatic OSA populations.
Another major issue is the therapeutic effectiveness of CPAP in these trials. CPAP adherence is a critical factor in therapeutic effectiveness. In the SAVE trial, the average nightly use was 3.3 hours, 20% of the subjects did not use CPAP at all, and only 42% used CPAP for at least 4 hours a night. The 4-hour threshold is an insurance-payment driven criterion widely enforced in the United States, which has gradually morphed into a clinical standard. The flaw in this sort of “criteria creep” is emphasized. Such a low adherence may have contributed to the null findings, as the residual apnea burden (see below) is substantial. Adherence to CPAP has modified efficacy in previous trials [145-147]: the higher the adherence, the better the outcome. A dose-response association between CPAP adherence and cardiovascular outcome was found in the SAVE and RIC-CADSA trials, in which CPAP adherence was related to a lower risk of stroke (HR, 0.56; 95% CI, 0.32 to 1.00) or cardiovascular endpoints (HR, 0.29; 95% CI, 0.10 to 0.86) [60,61]. However these findings came from secondary or on-treatment analysis, possibly biased by multiple comparisons or a healthy adherer effect [131,132]. In addition to adherence, the timing and the duration of CPAP-off periods affect therapeutic effectiveness [148-152]. The CPAP-off time is likely to predominate in the latter half of the night, when REM sleep is prevalent. OSA events during REM sleep are generally prolonged and associated with severe oxygen desaturation. Sleep apnea during REM, but not during NREM sleep, has been associated with hypertension, non-dipping of nocturnal blood pressure, and insulin resistance, even in subjects not considered to have OSA (AHI <5/hr) [153-156]. In recent observational finding from the Sleep Heart Health Study, severe REM OSA (AHI during REM sleep ≥30/hr) was associated with a higher incidence of cardiovascular events in the group with prevalent cardiovascular disease [157]. The cardiovascular effects of REM OSA have several important implications. The timing and duration of the CPAP-off periods as well as the subjects' sleep should be documented to define and interpret the effectiveness of intervention. The residual apnea burden, including REM OSA, is directly influenced by the proportion and timing of CPAP-on and -off periods [148-150]. In future trials, a predefined secondary analysis of REM OSA should be performed, considering its significant cardiovascular effects and prevalence [158]. REM OSA in subjects with AHI of <5/hr may bias the results of RCTs, especially secondary prevention trials [157]. From a diagnostic standpoint, simple cardiorespiratory monitoring devices, for example, a portable device consisting of airflow and oximetry, should not be used in future trials. Such a home kit cannot reliably detect REM OSA, central sleep apnea, and periodic limb movements (PLMs) [159]. The latter two conditions are not only commonly present in high risk populations for cardiovascular events (such as the elderly or subjects with established cardiovascular diseases or stroke), but also increase the cardiovascular risk of these individuals [160-164].
Finally, there is clear evidence that what is called OSA is a complex pathophysiological condition, with multiple and often interacting disease drivers [165-167]. These include upper airway collapsibility, impaired negative pressure response, reduced arousal threshold and high loop gain. The first three become irrelevant when the airway is adequately supported, but high loop gain will cause ongoing respiratory control instability and a high residual apnea burden or treatment intolerance. The risk of high loop gain is increased in those with cardiovascular comorbidities, setting the stage for impaired therapeutic responses [166]. Multi-modal therapy, such as low dose acetazolamide plus CPAP [168,169], may reasonably be considered in future clinical trials. The quality of data, specifically direct visualization of respiratory waveforms from current generation CPAP devices, can also detect residual disease burden and help identify those who could benefit from multi-modal therapy [170]. The question should not be whether CPAP is beneficial, but whether effective sleep therapy is beneficial.
Previous RCTs on cardiovascular outcomes usually adopted composite endpoints, mainly due to considerations of feasibility and statistical power. However, for future trials, therapeutic efficacy needs to be tested separately for each type of cardiovascular endpoint. Stroke is a primary candidate for this purpose. In CPAP trials and observational studies, OSA was more strongly related to stroke than to other cardiovascular diseases. In the SAVE trial, CPAP adherence led to the reduced risk of stroke (HR, 0.56; 95% CI, 0.32 to 1.00), and untreated OSA in women was associated with an increased incidence of cardiovascular events, particularly stroke (HR for stroke, 6.44; 95% CI, 1.46 to 28.3; and HR for coronary heart disease, 1.77; 95% CI, 0.76 to 4.09), which is concordant with the findings of the Sleep Heart Health Study concerning the relationship between OSA and stroke occurrence [49,60,171].
To summarize, we caution against discarding the benefit of CPAP therapy for the prevention of stroke and other cardiovascular events in OSA on the basis of results from the currently available RCTs. The overall findings suggest what really matters is the therapeutic effectiveness, which is determined by CPAP adherence, CPAP efficacy, apnea burden [148-150], and possibly disease phenotype [165-167]. Apnea burden is driven by residual or emergent sleep apnea during CPAP-on and-off periods along with the duration of CPAP-off periods [150-152,170]. In future trials for primary and secondary prevention in OSA, we need to adopt methods to determine the therapeutic effectiveness by measuring apnea burden, and to test whether effective therapy (defined by minimal or sufficiently low apnea burden, e.g., measured average AHI <5 over the entire treatment period including both CPAP-on and -off state in sleep) reduces the risk of stroke and other cardiovascular events.
Sleep duration and insomnia
Sleep duration
The relationship between sleep duration and stroke incidence is U-shaped in general; the risk for stroke is elevated in both short and long sleep groups [172-174]. In a recent meta-analysis (n=559,252), the pooled HR for stroke was 1.15 (95% CI, 1.07 to 1.24) for short sleep and 1.45 (95% CI, 1.30 to 1.62) for long sleep duration [51]. In another study, the pooled RR for stroke was 1.07 (95% CI, 1.02 to 1.12) and 1.17 (95% CI, 1.14 to 1.20) for each 1-hour decrease and increase in sleep duration, respectively [175].
Short sleep, commonly defined as <5 to 6 hours of nocturnal sleep, increases the risks of stroke, coronary heart disease, and death (Table 2) [52,173,175-180]. In a large-scale prospective study of older women (Women’s Health Initiative Study; age, 50 to 79 years; n=93,175), short sleep (≤6 hours) was associated with ischemic stroke (HR, 1.22; 95% CI, 1.03 to 1.44) in the group free from cardiovascular diseases and diabetes at the baseline [172]. Despite the large sample size, the study was limited by the participants’ characteristics, as enrollment was restricted to postmenopausal women. In the European Prospective Investigation into Cancer and Nutrition-Potsdam Study (n=23,620; age, 35 to 65 years; men 38.6%), subjects with short sleep (<6 hours) had a significantly increased risk for stroke (HR, 2.06; 95% CI, 1.18 to 3.59) during the 8-year follow-up [174]. In another population-based prospective study (n=17,604; age, 25 to 74 years; mean follow-up, 14 years), short sleep (≤5 hours) was significantly associated with stroke in men (HR, 1.44; 95% CI, 1.01 to 2.06) [173].
Long sleep duration (more than 9 hours of sleep) is also associated with stroke and cardiovascular mortality [51,178,179,181,182]. Qureshi et al. [182], using the First National Health and Nutrition Examination Survey (NHANES I), reported that stroke risk was higher in people who reported sleeping for >8 hours than for those who slept 6 to 8 hours (RR, 1.5; 95% CI, 1.1 to 2.1). In a recent population-based prospective study of 9,692 stroke-free participants, long sleep (HR, 1.46; 95% CI, 1.08 to 1.98), but not short sleep (HR, 1.18; 95% CI, 0.91 to 1.53), was associated with a higher risk of stroke [51].
The association between sleep duration and stroke mortality has been assessed in several prospective studies [178,183] and meta-analyses [52,175,184]. The Singapore Chinese Health Study (n=63,257) showed that long (≥9 hours) and short (≤5 hours) sleep duration (compared with 7 hours of sleep) were significantly associated with increased risk for total stroke mortality (HR, 1.54; 95% CI, 1.28 to 1.85; and HR, 1.25; 95% CI, 1.05 to 1.50, respectively) [178]. A meta-analysis showed that stroke mortality increased at either end of the sleep duration range [184]. However, other studies have found that the risk of stroke mortality increased only for long sleep duration, not for short sleep [52,175,181,183].
When interpreting the relationship between sleep duration and the risk of stroke or mortality, a few issues need to be considered. First, in most epidemiologic studies the assessment of sleep duration was based on self-report, not on objective measurement. Although self-reported sleep duration generally correlates to a degree with the duration measured by actigraphy or polysomnography [185,186], reported sleep duration is systematically over-estimated, especially in short sleepers, compared with objectively measured sleep duration [187]. Second, long sleep has a greater effect on mortality than short sleep [52,181,188], which can be explained by differences in the driving forces for each end of the sleep spectrum. In contrast to short sleep, which commonly results from voluntary sleep curtailment due to social requirements or individual preferences, long sleep indicates an increased need for sleep, especially in the elderly, influenced by comorbid medical conditions such as chronic devastating illness or inflammation [52,189-191]. Short sleep is a modifiable behavioral risk factor with a small but significant impact on cardiovascular morbidity and mortality. Long sleep is an indicator of comorbid medical conditions that confer higher mortality and vascular risk. A longitudinal observational study of the Whitehall II Cohort demonstrated the differential effects of short and long sleep: a decrease in sleep duration was associated with increased cardiovascular mortality, while an increase was related to non-cardiovascular death [192].
The linking mechanisms between short sleep and cardiovascular events include obesity, impaired glucose metabolism, hypertension, and dyslipidemia. Sleep loss contributes to the development of obesity or weight gain by disturbing the balance between energy intake and expenditure. Sleep deprivation leads to increased levels of the appetite stimulating hormone ghrelin and reduced levels of the anti-appetite hormone leptin [193,194]. Furthermore, reduced physical activity associated with sleep deprivation leads to weight gain by decreasing energy expenditure [195,196]. Short sleep is also associated with sympathetic overactivity [197], which leads to impaired glucose metabolism [180,198], hypertension, and non-dipping of blood pressure [176,199,200]. Sleep loss alters lipid metabolism [177,201,202]. Insufficient sleep activates inflammatory pathways, as indicated by increased levels of C-reactive protein and interleukin-6 [203,204].
Long sleep duration has been suggested as a potential marker for subsequent stroke risk [51,52,175,181,182]. The linking mechanisms between long sleep and stroke are still elusive, but increased inflammation and abnormal lipid profiles in long sleepers have recently been reported [202,205]. Long sleep has been associated with cardiovascular conditions including carotid artery atherosclerosis [206], atrial fibrillation [207,208], and white matter hyperintensities [209].
In summary, both short and long sleep duration are associated with a higher risk for stroke and mortality. Each end of the sleep duration spectrum has different implications. Short sleep confers an increased risk for cardiovascular events and mortality, via effects on blood pressure, glucose and lipid metabolism [52,175,210,211]. Long sleep is often an epiphenomenon of comorbidities that are commonly associated with increased sleep fragmentation, depressive symptoms, and poor general health [212]. The effects of sleep duration can have substantial long-term consequences, considering the increasing longevity of humans. Interventions such as sleep extension in short sleepers might reduce the cardiovascular risks. Simple measures to cope with sleep debt such as napping or weekend sleep extension might have a meaningful impact at the population level. Recent studies found that weekend catch-up sleep was associated with a lower risk of hypertension and obesity [213,214].
Insomnia
Insomnia is prevalent in approximately 10% to 20% of the adult population, with approximately 50% having a chronic form [215]. Chronic insomnia disorder is characterized by a complaint of difficulty initiating sleep and maintaining sleep, and waking up earlier than desired. The diagnosis of chronic insomnia requires occurrences on at least three nights per week for at least 3 months [216]. Insomnia was found to be a risk factor for cardiovascular events and death in several studies [190,217].
Sleep questionnaire-based studies have reported a significant association between insomnia symptoms and cardiovascular outcomes [218]. In a prospective study of 11,863 participants, subjective insomnia complaints were associated with an increased risk of hypertension and cardiovascular disease [219]. A large study of 21,438 subjects with insomnia and 64,314 matched controls found that the insomnia group had a 54% higher risk for stroke over a 4-year follow-up period than the control group [53]. However, these studies had the limitation that the diagnosis of insomnia was based solely on self-reported assessments or questionnaires for insomnia complaints, meaning that the sleep latency and time of being awake after sleep onset were generally overestimated.
Insomnia with objective short sleep duration (<5 hours on polysomnography), suggested as the most biologically vulnerable phenotype, carries a higher risk for impaired heart rate variability, hypertension, diabetes, neurocognitive impairment, and mortality, compared with insomnia with longer objective sleep (Table 3) [11,220-222]. Insomnia with objective short sleep may show better response to biological treatments, whereas insomnia with objective normal sleep may show better responses to psychological interventions than to biological treatments [11]. Elevated sympathetic and hypothalamic-pituitary-adrenal axis activity has been proposed as a mechanism for the cardiovascular effect of insomnia [11,220].
Periodic limb movements during sleep and restless legs syndrome
The defining feature of PLMS is periodic episodes of repetitive, highly stereotyped limb movements during sleep, which mostly occur in the lower extremities and can be associated with cortical arousal (Figure 3) [216]. The presence and severity of PLMS is determined by the average number of PLMs per hour of sleep (PLM index). PLM is commonly present in the general population (4.3% to 9.3% with PLM index >15/hr) and even more commonly in restless legs syndrome (RLS) patients (80% to 88% with PLM index >5/hr) [223,224]. PLM is not specific for RLS, as it is commonly seen with increasing age and in other medical disorders [224]. A positive relationship between PLM and cardiovascular events or mortality has been demonstrated in observational studies, and a greater risk attributed to PLM combined with arousals [55,164,225,226]. PLM with arousal induces an abrupt increase in blood pressure and heart rate through sympathetic overshoot [227,228].
Periodic limb movements during sleep. (A) Electroencephalography (EEG) plots for 30-second epoch. Arousal (arrowhead in panel A) is accompanied by periodic limb movement (arrowhead in panel B) during stage 2 rapid-eye-movement (REM) and non-REM sleep. The closed arrowheads in panels A and B indicate the same time. (B) Movement event plots for 2-minute epoch. Heart rate surges (open arrows) are associated with periodic brief electromyography (EMG) bursts in left or right tibialis anterior (close arrows). L, left; R, right; EOG, electrooculography; EKG, electrocardiogram; TA, tibialis anterior.
RLS is a chronic sensorimotor disorder characterized by an irresistible urge to move the limbs, which is usually worse at rest, occurs predominantly in the evening or night time, and is relieved by movement such as walking or stretching [216,229]. The prevalence of RLS in the general population has been reported to be between 5% and 10%, but it has been under-recognized [164]. RLS and its associated condition PLM may increase the risk for cardiovascular and cerebrovascular diseases [230]. Several studies showed that RLS might be a prognostic factor for stroke, although others obtained conflicting results (Table 3) [230-234]. In a large prospective cohort study, RLS tended to be associated with an elevated risk of total and cardiovascular mortality, and this association between RLS and mortality increased in women with a longer duration of RLS diagnosis [235,236]. However, two meta-analyses assessing RLS as a risk factor for incident cardiovascular events and all-cause mortality were inconclusive [163,164]. More severe, longer duration exposures, and secondary forms of RLS were associated with increased risk for stroke [164,230,237].
Sympathetic overactivity, metabolic dysregulation, inflammation, oxidative stress, peripheral hypoxia, and hypothalamicpituitary-adrenal activation have been proposed as possible linking mechanisms between PLM/RLS and cardiovascular diseases [238]. Repeated nocturnal fluctuations in heart rate and blood pressure that are associated with PLM and related microarousals cause daytime hypertension, subsequently increasing the risk for cerebrovascular diseases [227,228]. In a prospective study of 3,116 elderly men, PLM increased atrial fibrillation risk in age-dependent manner [239].
Circadian rhythm disorders and stroke
Circadian rhythms are endogenous biological rhythms with near-24-hour periodicity. The internal near-24-hour circadian pacemaker is entrained to the 24-hour light-dark cycle by exogenous cues (primarily light/dark cues, but also eating, and to lesser degrees exercise and social interactions) [216]. Shift workers are vulnerable to disruption of normal circadian rhythms. Shift work sleep disorder is characterized by complaints of excessive sleepiness or insomnia that occur when work hours overlap with the usual sleep time. Night shift work is associated with significant interference with the endogenous nocturnal blood pressure decline, resulting in abnormally elevated blood pressure during the shift that persists into the following day [57]. Shift workers are at risk for obesity, hypertension, diabetes, cardiovascular disease, and overall mortality [240-243]. In a cohort study of 80,108 nurses, rotating night shift work was associated with a 4% increased risk of ischemic stroke for every 5 years of exposure, after adjusting for standard vascular risk factors [58]. A recent systematic meta-analysis demonstrated that shift work was associated with ischemic stroke (RR, 1.05; 95% CI, 1.01 to 1.09), although the original studies showed mixed results [244]. Further studies should attempt to clarify the relationship between circadian rhythm disorders (including shift work sleep disorder) and stroke risk, as shift work is common and likely to increase.
Conclusions
Sleep disorders are highly prevalent in patients at risk for stroke, and may be modifiable risk factors for stroke. OSA increases the risk of stroke independently, but the reported lack of therapeutic effectiveness of CPAP for stroke prevention and cardiovascular protection should be cautiously interpreted. New clinical trials with improved therapeutic precision are necessary. Short or long sleep duration, and insomnia with objective short sleep duration, could be risk factors for stroke and mortality. Sleep-related movement disorders, including PLMS and RLS, are also potential risk factors for stroke. The overall findings suggest that systematic screening and proper management of sleep disturbances can substantially contribute to stroke risk modification at the population level.
Notes
Disclosure
Robert J. Thomas is co-inventor and patent holder of the ECG-derived sleep spectrogram, which may be used to phenotype sleep quality and central/complex sleep apnea. The technology is licensed by Beth Israel Deaconess Medical Center to MyCardio, LLC. He is also co-inventor and patent holder of the Positive Airway Pressure Gas Modulator, being developed for treatment of central/complex sleep apnea. He is a consultant in software development for DeVilbiss. The other authors have no financial conflicts of interest.