Gray-Matter Volume Estimate Score: A Novel Semi-Automatic Method Measuring Early Ischemic Change on CT
Article information
Abstract
Background and Purpose
We developed a novel method named Gray-matter Volume Estimate Score (GRAVES), measuring early ischemic changes on Computed Tomography (CT) semi-automatically by computer software. This study aimed to compare GRAVES and Alberta Stroke Program Early CT Score (ASPECTS) with regards to outcome prediction and inter-rater agreement.
Methods
This was a retrospective cohort study. Among consecutive patients with ischemic stroke in the anterior circulation who received intra-arterial therapy (IAT), those with a readable pretreatment CT were included. Two stroke neurologists independently measured both the GRAVES and ASPECTS. GRAVES was defined as the percentage of estimated hypodense lesion in the gray matter of the ipsilateral hemisphere. Spearman correlation analysis, receiver operating characteristic (ROC) comparison test, and intra-class correlation coefficient (ICC) comparison tests were performed between GRAVES and ASPECTS.
Results
Ninety-four subjects (age: 68.7±10.3; male: 54 [54.9%]) were enrolled. The mean GRAVES was 9.0±8.9 and the median ASPECTS was 8 (interquartile range, 6-9). Correlation between ASPECTS and GRAVES was good (Spearman’s rank correlation coefficient, 0.642; P<0.001). ROC comparison analysis showed that the predictive value of GRAVES for favorable outcome was not significantly different from that of ASPECTS (area under curve, 0.765 vs. 0.717; P=0.308). ICC comparison analysis revealed that inter-rater agreement of GRAVES was significantly better than that of ASPECTS (0.978 vs. 0.895; P<0.001).
Conclusions
GRAVES had a good correlation with ASPECTS. GRAVES was as good as ASPECTS in predicting a favorable clinical outcome, but was better than ASPECTS regarding inter-rater agreement. GRAVES may be used to predict the outcome of IAT.
Introduction
Early ischemic changes (EIC) on Computed Tomography (CT) represent early cytotoxic edema and possible development of irreversible tissue injury [1,2]. As reperfusion to irreversibly injured tissue is not beneficial and can even be harmful if it leads to hemorrhage, European Cooperative Acute Stroke Study trials have excluded patients with EIC involving more than a third of the middle cerebral artery territory on CT [3-5]. However, this method is not reliable even by experienced clinicians [6]. Therefore, to improve the reliability of EIC measurement, the Alberta Stroke Program Early CT Score (ASPECTS), a systematic method quantifying EIC, was devised [7]. ASPECTS has proved its worth in predicting outcomes of reperfusion therapy in acute ischemic stroke [7-12], and was used to exclude patients with large infarct core in the recently published trials which showed clear benefit of intra-arterial therapy (IAT).
Nevertheless, highly trained personnel are required to measure ASPECTS and there have been conflicting reports of inter-rater agreement [7,13-17]. Therefore, we have developed a novel method named Gray-matter Volume Estimate Score (GRAVES), which is expected to have better reliability as it is a semi-automatic method of measuring EIC on CT using computer software. The object of this study was to compare GRAVES and ASPECTS with regards to outcome prediction and inter-rater agreement
Methods
Patients
This was a retrospective cohort study. Candidates for this study were consecutive patients with ischemic stroke in the unilateral anterior circulation who received IAT between January 2009 and May 2012, at our institute. All those with a readable baseline CT were included. The local institutional review board approved this study and granted a waiver of consent because of its retrospective nature.
Reperfusion therapy protocol and follow-up
Our reperfusion therapy and imaging protocols have been published previously [18,19]. Briefly, patients presenting within 3 hours of stroke onset were treated with intravenous tissue plasminogen activator (IV tPA) (Actylase; Boehringer-Ingelheim, Ingelheim, Germany) at a standard dose of 0.9 mg/kg (10% as bolus then 90% as an infusion for 60 minutes) after Non-contrast Computed Tomography (NCCT). If patients showed an unsatisfactory clinical response (defined as a <50% improvement as measured by the National Institutes of Health Stroke Scale [NIHSS]) at the end of IV tPA infusion, IAT was considered after follow-up NCCT with CT angiography. Those patients who presented between 3-6 hours of stroke onset were also considered for IAT after NCCT with CT angiography. Reperfusion was graded by the Thrombolysis in Cerebral Infarction scale. Successful reperfusion was defined as Thrombolysis in Cerebral Infarction 2b or 3. The modified Rankin Score was assessed at 3 months after stroke onset. Favorable outcome was defined as modified Rankin Score ≤2 at 3 months.
Imaging acquisition
NCCT images were obtained on a multi-detector row CT system (Sensation 64; Siemens, Erlangen, Germany or LightSpeed Plus; GE Healthcare, Milwaukee, WI, USA). The sequential axial 3-mm NCCT (Sensation 64) was performed with the following parameters: 120 kVp, 300 mAs, field of view of 250 mm, 1 s/rotation, 30×0.6 mm collimation and a H30s medium reconstruction kernel. The sequential axial 5-mm NCCT (LightSpeed Plus) was performed with the following parameters: 120 kVp, 250 mAs, field of view of 250 mm, 0.8 s/rotation, 4×1.25 mm collimation, and a smooth reconstruction kernel.
NCCT analysis using GRAVES and ASPECTS
To measure EIC, CT scans taken just before IAT were analyzed. Two stroke neurologists independently reviewed CT scans and measured GRAVES and ASPECTS blinded to clinical information except stroke side. Any discrepancy between the two readers was resolved by reaching a consensus.
GRAVES was developed based on the software platform for image analysis (Xelis; INFINITT Healthcare, Seoul, Korea). GRAVES was defined as the estimated percentage of hypodense lesion in the gray matter of symptomatic hemisphere. With an assumption that contralateral hemisphere is normal and that two hemispheres are relatively symmetric, GRAVES was measured by subtracting estimated area of normal gray matter in the symptomatic hemisphere (GMsymptomatic) from that in the contralateral hemisphere (GMcontralateral; GRAVES=[GMcontralateral-GMsymptomatic]/[GMcontralateral]×100). Estimated area of normal gray matter was operationally defined as an area of which Hounsfield unit (HU) is between the mean HU of the contralateral normal caudate head (HUcaudate) and HUcaudate+9. In case of an abnormal contralateral caudate head, another normal structure of the contralateral deep gray matter such as the thalamus or lentiform nucleus was selected, at the discretion of the rater. To reflect area asymmetry between the hemispheres, estimated areas of normal brain parenchyme in each hemisphere (Parenchymesymptomatic and Parenchymecontralateral) predefined as area of which HU is between 20 and 55, were used for adjustment (Adjusted GMcontralateral= GMcontralateral*[Parenchymesymptomatic/Parenchymecontralateral]). CT slices that cover 60 mm from upper midbrain (superior colliculus) level were all included for analysis (e.g. 20 axial slices in 3-mm NCCT, and 12 axial slices in 5-mm NCCT). To measure GRAVES, raters set the range of axial CT slices to analyze, adjusted the central line separating the hemispheres, and drew the ROI on the contralateral caudate head. After the stated simple steps, computer software calculates GRAVES automatically and reports immediately (Figure 1).
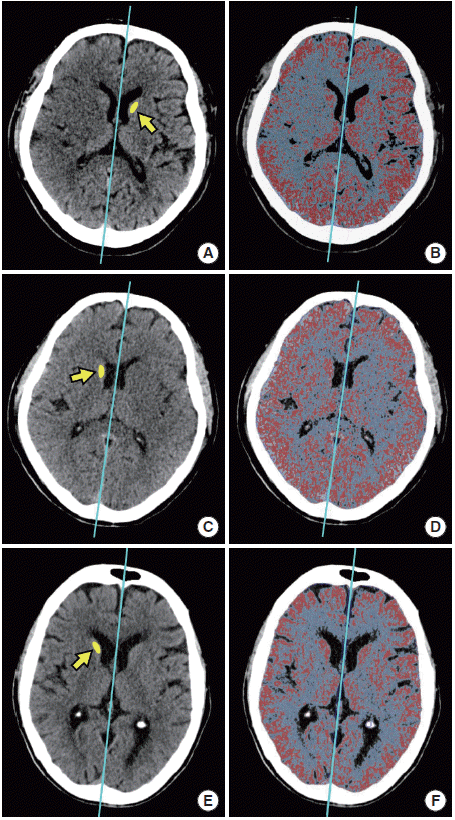
Representative figures of GRAVES measurements. Representative measurements of GRAVES in a patient with large core (GRAVES, 19.75; ASPECTS, 1; A and B), moderate core (GRAVES, 12.93; ASPECTS, 6; C, D), and small core (GRAVES, 1.92; ASPECTS, 10; E and F) are shown. Central lines that separate the hemispheres are drawn automatically and can be adjusted by a rater. The reference values of Hounsfield unit (HU) for the gray matter are determined by drawing a region of interest on the contralateral caudate head (yellow-colored area indicated arrow; A, C, and E). Then, computer software automatically analyzes the mean HU of region of interest. Red colored pixels are the area of which the values of HU are between the mean HU of the contralateral caudate head (HUcaudate) and HUcaudate + 9. These represent estimated normal gray matter. Blue-colored pixels are the area of which the values of HU are between 20 and 55. These represent estimated normal brain parenchyme (B, D, and F). GRAVES, Gray-matter Volume Estimate Score; ASPECTS, Alberta Stroke Program Early CT Score.
ASPECTS was measured following recently modified definition of EIC [20]. Tissue hypoattenuation and loss of gray–white matter differentiation, but not isolated cortical swelling, were taken as evidence for EIC. All axial CT images were used and settings for the window level were adjusted at the discretion of the rater to increase contrast between normal and ischemic brain areas.
Statistical analysis
Values were presented as number (%), mean±standard deviation or median (interquartile range [IQR]) as appropriate. Univariate analyses (independent sample t-test or Mann-Whitney U test for continuous variables and χ2 test or Fisher’s exact test for categorical variables as appropriate) were performed to compare baseline characteristics, treatment modalities, time parameters, and imaging characteristics between the favorable and the unfavorable outcome groups. Variables achieving a P value less than 0.05 in the univariate analyses for favorable outcome were adjusted for the multivariate analyses (binary logistic regression models with favorable outcome as the dependent variable). Spearman correlation analysis between ASPECTS and GRAVES was performed. Receiver operating characteristic (ROC) comparison test was performed to compare predictability of a favorable outcome between ASPECTS and GRAVES. The Youden index was used to determine the optimal cut-off value of ASPECTS and GRAVES. Inter-rater agreements were assessed with linear-weighted κ statistics, intra-class correlation coefficient (ICC) and Bland-Altman plot. ICC comparison test was performed to compare inter-rater agreement of ASPECTS and that of GRAVES. Statistical analyses were performed using the IBM SPSS Statistics for Windows Version 20.0 (IBM Corp., Armonk, NY, USA) and MedCalc (MedCalc, Mariakerke, Belgium). A two-sided P value of <0.05 was considered statistically significant.
Results
Patients enrollment
A total of 250 patients with acute ischemic stroke received IV tPA and/or IAT at our institute between January 2009 and May 2012. Of these, 155 who received IAT were considered for inclusion in this study. Among the 155 patients, we excluded 25 with posterior circulation infarction, 3 with bilateral infarctions, 8 with unavailable modified Rankin Score at 3 months, 2 patients with unavailable baseline CT and 23 with unreadable CT (12 with old territorial infarction, 10 with motion artifact, and 1 with brain tumor). Finally, 94 patients were enrolled in this study.
Baseline characteristics
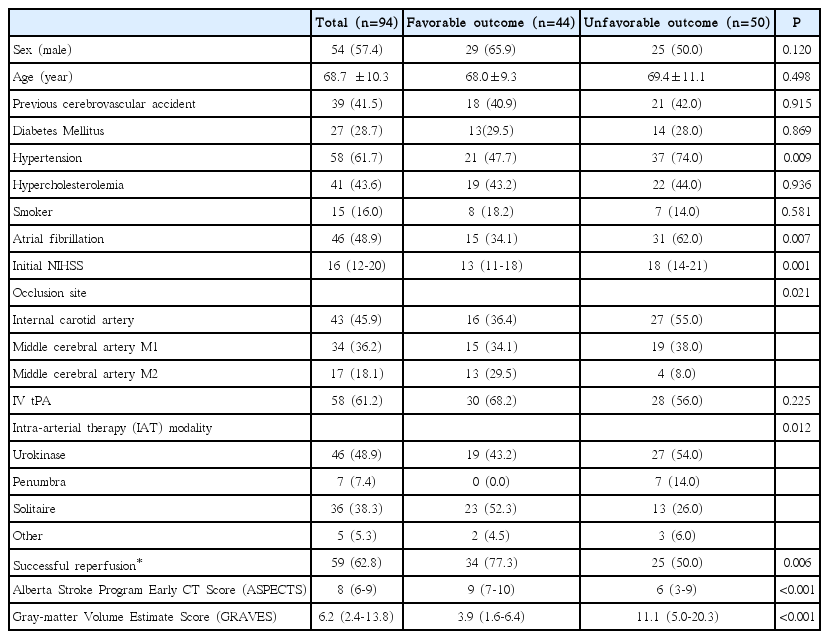
Mean age of the study subjects was 68.7±10.3 years and 54 (57.4%) were men. The median NIHSS score at admission was 16 (IQR, 12-20). The median GRAVES of all subjects, internal carotid artery occlusion, middle cerebral artery M1 occlusion, and M2 occlusion was 6.2 (IQR, 2.4-13.8), 8.6 (IQR, 2.4-15.5), 6.1 (IQR, 2.5-16.0), and 5.0 (IQR, -0.5-9.5), respectively. The median ASPECTS of all subjects, internal carotid artery occlusion, middle cerebral artery M1 occlusion, and middle cerebral artery M2 occlusion was 8 (IQR, 6-9), 7 (IQR, 3-9), 8 (IQR, 6-10), and 9 (IQR, 7-10), respectively. We treated 58 (61.2%) patients with combined IV tPA and IAT, and 36 (38.8%) with IAT only. Successful reperfusion was achieved in 59 (62.8%) patients, and 44 (48.9%) had a favorable outcome.
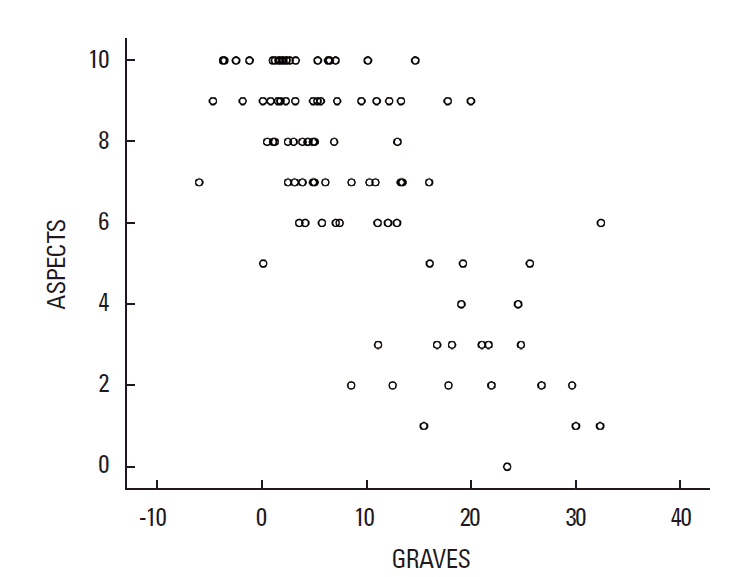
Correlation between GRAVES and ASPECTS
Correlation between GRAVES and ASPECTS was good (Spearman’s rank correlation coefficient, -0.642; P<0.001; Figure 2).
Outcome prediction of GRAVES and ASPECTS
The favorable outcome group had significantly less hypertension, less atrial fibrillation, lower NIHSS, more initial M2 occlusion, more initial IAT with Solitaire, more successful reperfusion, lower GRAVES, and higher ASPECTS compared to the unfavorable outcome group in the univariate analyses (Table 1). In the multivariate analysis adjusting for hypertension, atrial fibrillation, NIHSS, initial occlusion site, initial IAT modality and successful reperfusion, not only ASPECTS (Odds Ratio [OR], 1.401; 95% Confidence Interval [CI], 1.070-1.833; P=0.014), but also GRAVES (OR, 0.878; 95% CI, 0.795-0.970; P=0.011) were independently associated with a favorable outcome.
ROC comparison analysis showed that the predictive value of GRAVES for favorable outcome (area under curve [AUC], 0.765; 95% CI, 0.666-0.846) was not significantly different from that of ASPECTS (AUC, 0.717; 95% CI, 0.615-0.805; P=0.308; Figure 3A). The optimal cutoff value of ASPECTS to predict a favorable outcome was >7 (Youden index, 0.37; Sensitivity, 72.7%; Specificity, 64.0%), while that of GRAVES was ≤6.5 (Youden index, 0.47; Sensitivity, 77.3%; Specificity, 70.0%). When we performed ROC comparison analysis for only 59 patients with successful reperfusion, the predictive value of GRAVES for a favorable outcome (AUC, 0.762; 95% CI, 0.634-0.862) was similar to that of ASPECTS (AUC, 0.744; 95% CI, 0.613-0.848; P=0.776; Figure 3B). The predictive value of a multivariate model for a favorable outcome including GRAVES (with age, sex, hypertension, atrial fibrillation, initial occlusion site, NIHSS, Solitaire, and successful reperfusion; AUC, 0.899; 95% CI, 0.819-0.951) was similar to that of a multivariate model including ASPECTS (AUC, 0.907; 95% CI, 0.829-0.957; P=0.552; Figure 3C).
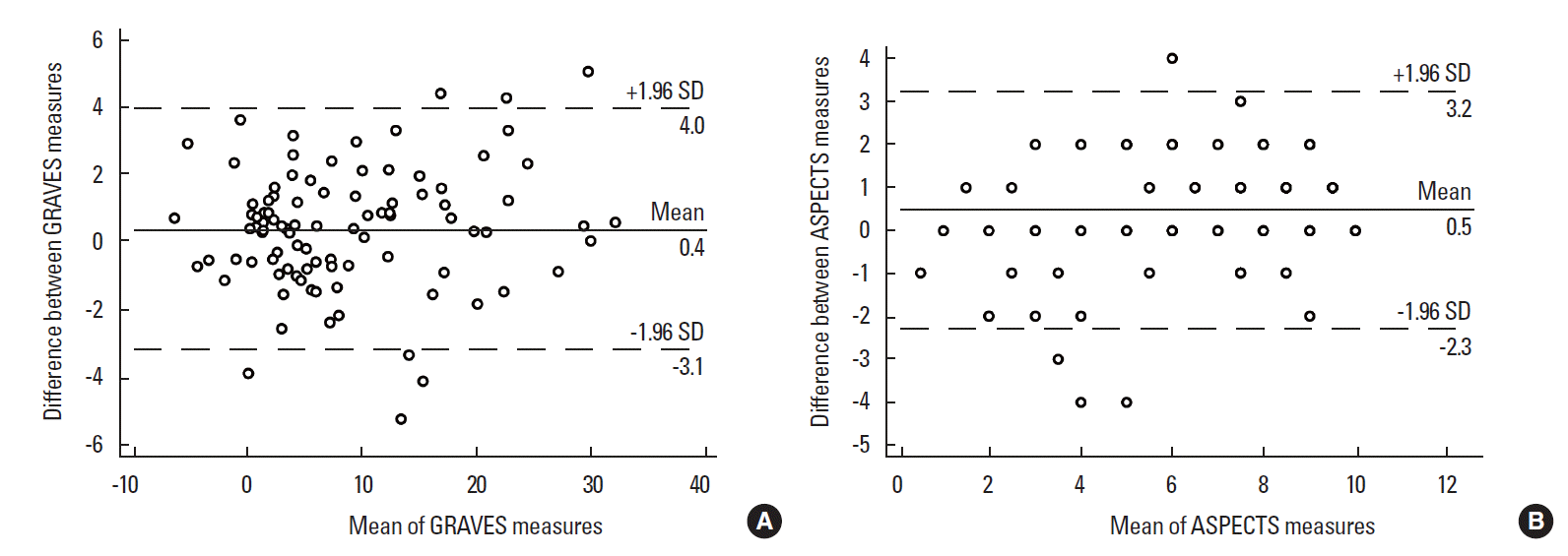
Reliability of GRAVES and ASPECTS
Linear-weighted κ of ASPECTS was 0.662 (95% CI, 0.595-0.728). ICC of ASPECTS was 0.895 (95% CI, 0.822-0.935). ICC of GRAVES was 0.978 (95% CI, 0.966-0.985). ICC comparison analysis revealed that inter-rater agreement of GRAVES was significantly better than that of ASPECTS (P<0.001). Bland-Altman plot showed that the mean difference between two measures of GRAVES was 0.4 (95% limits of agreement, -3.1-4.0) and that of ASPECTS was 0.5 (95% limits of agreement, -2.3-3.2). There were no significant trend between the difference and the mean of two measures for both GRAVES and ASPECTS (Figure 4).
Discussion
A rapid, easy, and reliable determination of irreversibly damaged brain tissue is critical in selecting candidates for reperfusion therapy in acute stroke patients. Occlusion of the cerebral artery causes a decrease in cerebral blood flow to the downstream territory, which subsequently results in deprivation of glucose and oxygen, depletion of adenosine triphosphate, changes in ionic homeostasis, intracellular accumulation of water, and cell death in the core region of ischemia. As HU of CT is inversely correlated with the amount of water, an area of low-density lesion represents an irreversibly damaged area with cytotoxic edema [1,2]. Irreversible damage is usually more prominent in the gray matter during the very early stage of infarction because gray matter is more vulnerable to ischemic insult compared to white matter [21]. Classic early CT signs detailed in the literature including loss of insular ribbon, obscuration of lentiform nucleus and loss of gray-white matter differentiation [2] all imply ischemic insult of gray matter. Thus, we have devised a simple method to quantify the extent of EIC by calculating the estimated percentage of hypodense lesion in the gray matter of the symptomatic hemisphere. To reflect individual difference of density in the gray matter, we calculated mean HU of contralateral caudate head and used it as a reference value of estimated normal gray matter. While the means of HU in caudate head were different in each patient, the standard deviations were relatively consistent between 4 and 5. Therefore, we defined the range of reference representing normal gray matter as pixels of which HU are between the mean HU of the contralateral caudate head (HUcaudate) and HUcaudate+9 which accounts for two standard deviations. We chose the caudate head because it is relatively easy to distinguish and usually not affected by bone-induced streak artifact. The area of which HU is within the individually defined thresholds is not exclusively limited to normal gray matter. However, the areas of which HU is within the thresholds outside of normal gray matter is relatively small and symmetric between the hemispheres, and we operationally define the area of which HU is within the thresholds as the estimated area of normal gray matter. In addition, the estimated areas of normal brain parenchyme in each hemisphere were used for adjustment because the normal volume of the bilateral gray matter could be asymmetrical. While the thresholds of the normal gray matter were defined individually, lower and upper thresholds of HU for normal brain parenchyme were predefined as 20 and 55 respectively to enable rapid measurement.
It was not surprising that GRAVES was well correlated with ASPECTS given the fundamental principle of both GRAVES and ASPECTS, which were designed to estimate the EIC in the gray matter. Outcome predictability of GRAVES was also similar to that of ASPECTS, not only in the univariate model and the multivariate model, but also in the univariate model with successfully reperfused patients. GRAVES may also be used for patient selection of reperfusion treatment as ASPECTS is. However, further external validation studies are required to use GRAVES for this purpose in clinical practice. Optimal cutoff value of ASPECTS for predicting favorable outcome was >7 in this study, which was consistent with previously reports [7]. Although further validation is required, GRAVES ≤6.5 was the optimal cutoff value for predicting favorable outcome in this study.
When calculating GRAVES with computer software, raters need only to select the range of CT slices to analyze, adjust the central line to separate the hemispheres, and draw ROI on the contralateral caudate head, which can usually be done approximately within 3 minutes. Thus, raters who have a basic understanding of brain anatomy can measure GRAVES without difficulty and inter-rater agreement of GRAVES was excellent. Compared to ASPECTS which requires certain level of expertise, GRAVES may have an advantage in this regard.
This study has several limitations. First, selection bias is unavoidable considering that this study was a retrospective analysis of an IAT cohort and the sample size was only modest. Second, GRAVES could not be measured in patients with previous infarction or other structural lesions in the contralateral gray matter because it presumes the normality of the contralateral hemisphere. It was also sensitive to motion artifact. In this study, 12 patients with old territorial infarction and 10 patients with severe motion artifact who otherwise met inclusion criteria were excluded. Third, GRAVES is only applicable to patients with anterior circulation infarction.
Conclusions
GRAVES has a good correlation with ASPECTS. GRAVES was as good as ASPECTS in predicting a favorable clinical outcome, but was better than ASPECTS with regard to inter-rater agreement in patients who received IAT. Although further validation with larger sample size date is required, GRAVES and ASPECTS might be used interchangeably or alternatively to predict outcomes and aid the selection of patients for reperfusion therapies.
Acknowledgements
The authors thank Hanyoung Kim and Chang Min Shin for their excellent technical contribution to software development.
Notes
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health & Welfare Affairs, Republic of Korea (HI08C2149).
Notes
The authors have no financial conflicts of interest.