Perfusion Profiles May Differ Between Asymptomatic Versus Symptomatic Internal Carotid Artery Occlusion
Article information
Dear Sir:
In the clinical setting, it can be challenging to discern whether an internal carotid artery occlusion (ICAO) is acute or chronic. Knowledge of the respective perfusion characteristics holds valuable implications for assessing the collateral status or interventional strategy [1]. This study aims to assess what distinct features on cerebral magnetic resonance (MR) perfusion can differentiate acute symptomatic from chronic asymptomatic ICAO.
Patients with unilateral ICAO who underwent MR perfusion were retrospectively enrolled. Chronic asymptomatic ICAO cases, having no stroke/transient ischemic attack history for at least 1 year before magnetic resonance imaging (MRI), were collected from Chang Gung Memorial Hospital (Taiwan) database (August 2013 to July 2017). Acute symptomatic ICAO cases, defined by acute stroke symptoms within 24 hours before MRI, were collected from Stanford Stroke Center’s clinical database (2012–2018) and the DEFUSE 2 (Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2) and DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3) trials [2,3]. Those with poor MRI quality or >50% contralateral internal carotid artery or middle cerebral artery (MCA) stenosis were excluded. The study protocol was approved by the Institutional Review Board of the Chang Gung Memorial Hospital (IRB No.: 202200342B0). Informed consent was obtained or waived by the local institutional review board.
MR perfusion maps (time-to-maximum [Tmax], mean transit time [MTT], cerebral blood flow [CBF], cerebral blood volume [CBV]) were generated using RAPID software (Ver 4.9; iSchema-View, Menlo Park, CA, USA) and analyzed both quantitatively and qualitatively. Quantitative measures included perfusion lesion volume on the Tmax map at four thresholds (>4 s, >6 s, >8 s, >10 s) in the affected hemisphere and the hypoperfusion intensity ratio (HIR), indicating collateral perfusion status (Tmax >10 s lesion volume/Tmax >6 s lesion volume) [4]. Ipsilateral collaterals were assessed on MR angiography using the circle of Willis (CoW) collateral score (range 0–4, higher score indicating better collaterals) [5]. Qualitative perfusion interpretations were performed by a stroke neurologist (LVO) and a neuroradiologist (JJH) rating the affected hemisphere as decreased, symmetric, or increased on each map (Tmax, MTT, CBF, CBV). Inter-rater differences were resolved through a consensus meeting. Using qualitative ratings, a decision algorithm distinguishing acute symptomatic from chronic asymptomatic ICAO was developed. Three raters (GWA, MMH, SL) applied this algorithm to determine each patient’s ICAO status (symptomatic versus asymptomatic).
Demographics were compared using chi-square and t-tests. Perfusion volume and CoW collateral differences were assessed using the Mann-Whitney U test. The diagnostic value of quantitative parameters was evaluated with receiver operating characteristic curves. The test characteristics of qualitative ratings of the perfusion maps included accuracy, sensitivity/specificity, and negative/positive predictive value (NPV/PPV) for diagnosing acute symptomatic ICAO. Inter-rater reliability was determined using Gwet’s AC1 by SAS Ver. 9.4 (SAS Institute, Cary, NC, USA). Other statistical analyses employed SPSS Ver. 24 (IBM Corp., Armonk, NY, USA). A P value <0.05 indicated statistical significance.
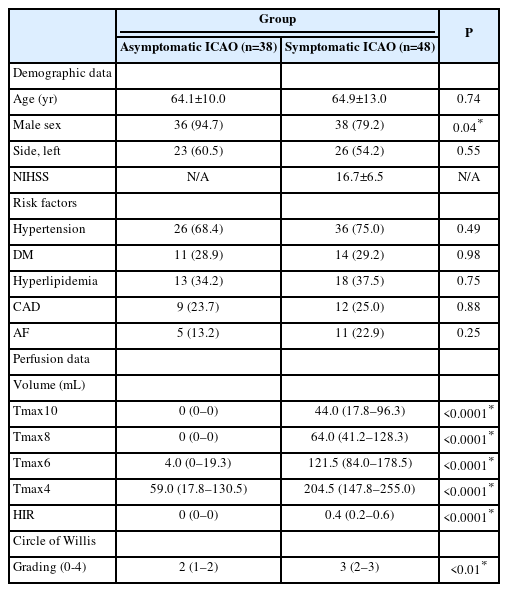
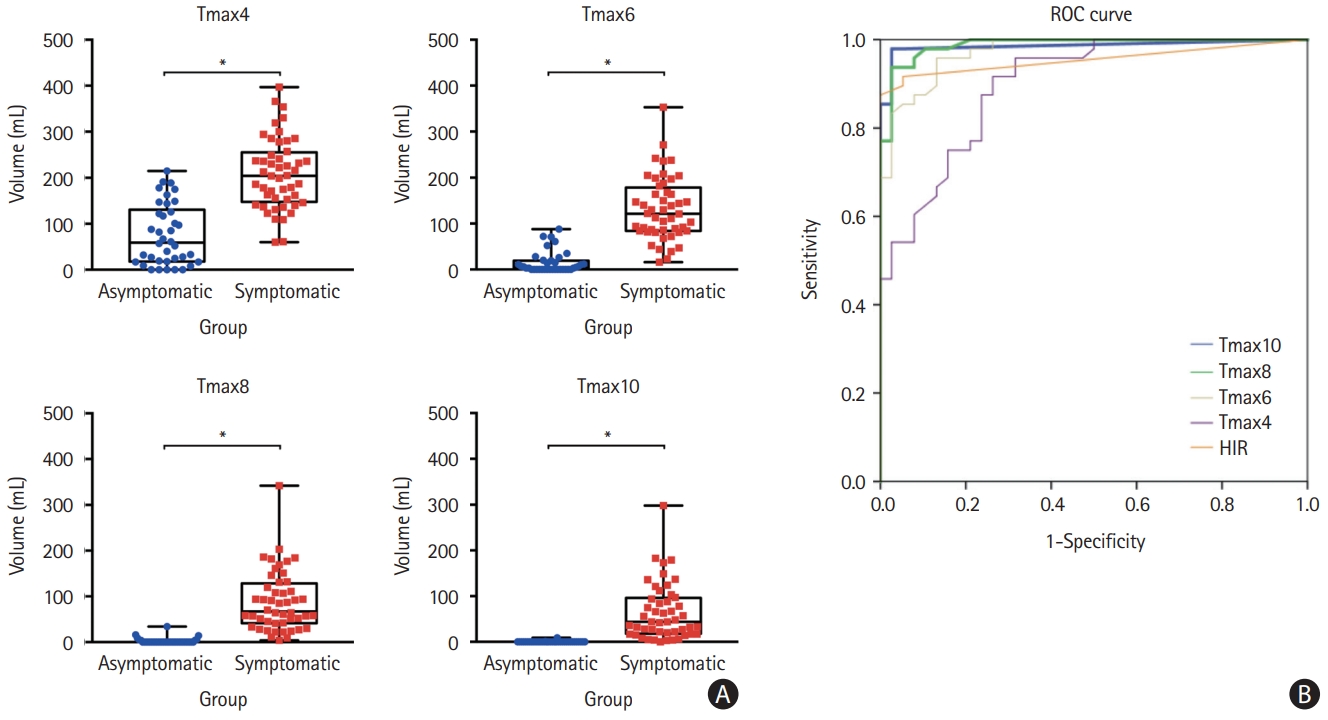
This study included 38 asymptomatic and 48 symptomatic ICAO patients (Supplementary Figure 1). Baseline characteristics were similar between groups, except for a higher percentage of men among asymptomatic cases (Table 1). Quantitative perfusion analysis showed larger Tmax delay volumes and higher HIR in symptomatic ICAO patients, indicating worse collaterals (P<0.0001 for all comparisons) (Table 1). Tmax >10 s volume was the best discriminator (area under the curve=0.99, 95% confidence interval [CI]=0.96–1.00) (Figure 1); a Tmax >10 s volume >2 mL predicted symptomatic occlusion with 97.9% sensitivity, 97.4% specificity, 97.9% PPV, and 97.4% NPV (Supplementary Table 1). The CoW collateral grade was lower among acute symptomatic patients (median 2 vs. 3, P<0.01) (Table 1). Qualitative assessments showed that acute symptomatic ICAO patients typically displayed delayed Tmax (100%), prolonged MTT (97.9%), and decreased CBF (81.2%), with varying CBV patterns (Supplementary Table 1, Supplementary Figures 2 and 3A). Prolonged MTT was the optimal metric for differentiating acute from chronic ICAO with accuracy, sensitivity, specificity, PPV, and NPV all exceeding 97%. Chronic asymptomatic ICAO cases typically demonstrated delayed Tmax (60.5%) with symmetrical MTT (97.4%), CBF (94.7%), and CBV (94.7%) (Supplementary Figures 2 and 3B). Remarkably, the only asymptomatic patient with prolonged MTT experienced an ipsilateral ischemic stroke 6 months later. A decision algorithm, based on Tmax, MTT, and CBF (Figure 2A), achieved 96.5%–98.8% accuracy in differentiating symptomatic from asymptomatic ICAO (Figure 2B). Inter-rater AC1 for the three raters was 0.89 (95% CI 0.82–0.97).
Quantitative perfusion measurements. (A) Box plots showing the comparisons of Tmax (>4 s, >6 s, >8 s, and >10 s) volumes between patients with asymptomatic (blue) and symptomatic (red) internal carotid artery occlusions (ICAO). Patients with symptomatic occlusions had larger Tmax lesion volumes for each Tmax threshold (*P<0.01). (B) Receiver operating characteristic (ROC) curves for the four Tmax thresholds and for the hypoperfusion intensity ratio (HIR, orange) to distinguish symptomatic from asymptomatic ICAO. The largest area-under-curve (AUC) was observed for the curves of Tmax10 (blue; AUC=0.99, 95% confidence interval (CI)=0.96–1.00) and Tmax8 (green; AUC=0.98, 95% CI=0.97–1.00).
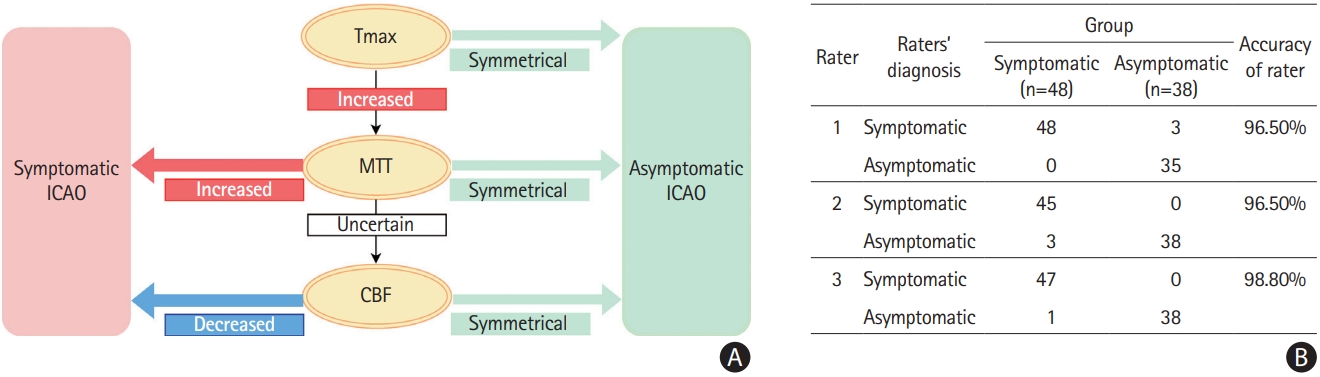
Decision algorithm for classification. (A) Decision tree to differentiate symptomatic from asymptomatic internal carotid occlusion (ICAO) based on magnetic resonance perfusion characteristics. (B) Raters’ ability to differentiate asymptomatic and symptomatic patients according to the decision tree. MTT, mean transit time; CBF, cerebral blood flow.
This study reveals distinct patterns on MR perfusion differentiating acute symptomatic from chronic asymptomatic ICAO. A Tmax >10 s lesion exceeding 2 mL is the optimal quantitative measure for identifying acute symptomatic cases (sensitivity and specificity >95%). On a qualitative perfusion review, the presence of prolonged MTT is the ideal metric for identifying acute symptomatic ICAO (sensitivity and specificity >95%).
Severely prolonged Tmax is a marker of poor collateral circulation. We have previously shown higher HIR (Tmax >10 s/Tmax >6 s) indicating poorer collaterals [4]. Others have shown severe Tmax delay (>16 s) corresponding to poor collaterals in acute MCA occlusions [6]. The current study’s finding that acute symptomatic ICAO is associated with Tmax >10 s delay, aligns with prior studies revealing worse collaterals in acute ICAO compared to chronic ICAO [7]. Whereas profound Tmax delay is specific for acute ICAO, the absence of any Tmax delay (symmetric Tmax map) was 100% specific for identifying asymptomatic ICAO in our cohort. Milder Tmax delays (4–6 s) were commonly observed in both groups and held no discriminatory value.
On qualitative perfusion maps review, MTT was the most effective discriminator. Prolonged MTT of the affected hemisphere was both highly specific and sensitive for acute symptomatic ICAO, consistent with previous reports [8], while asymptomatic ICAO was characterized by a symmetrical MTT. Notably, one asymptomatic patient who had prolonged MTT developed a stroke 6 months after the MRI. This finding suggests that the presence of prolonged MTT might be a marker for increased stroke risk among asymptomatic ICAO patients. Importantly, the MTT calculation in the software employed here is delay-insensitive [9], which may explain why our results differ from earlier studies that utilized delay-sensitive MTT algorithms and reported either symmetrical or prolonged MTT in asymptomatic ICAO patients [8].
The decision algorithm distinguishing symptomatic from asymptomatic ICAO incorporated Tmax, MTT, and CBF (Figure 2A) maps. CBV was not included because it did not add discriminatory power. Raters, blinded to clinical information, classified cases using the algorithm with high accuracy and strong inter-rater agreement.
While the excellent diagnostic performance of our discriminators is partly attributed to the clinical phenotype differences between the two groups, our study provides useful information in scenarios where patients presenting with ICAO manifest vague or mild acute symptoms. Furthermore, perfusion profile enhances the detection of compromised hemodynamics in asymptomatic cases.
Our study has notable limitations. First, the perfusion patterns associated with symptomatic and asymptomatic ICAO were validated internally, requiring further external validation in different cohorts. Second, our study focused on MR perfusion, whereas computed tomography (CT) perfusion is more prevalent in acute stroke assessment. Though our findings may apply to CT perfusion, confirmation through other studies is needed. Third, chronic ICAO is probable exclusively caused by atherosclerosis, whereas acute ICAO might be related to either atherosclerosis or embolism. Future studies are needed to investigate if perfusion profiles differ according to stroke etiology. Fourth, ICAO-related stroke can be caused by cerebral hypoperfusion or embolism from the occluded artery [10]. It is possible that normal MTT and the absence of severe Tmax prolongation among asymptomatic ICAO patients reflects a low risk for stroke secondary to hypoperfusion, but is unrelated to embolism risk.
In conclusion, our study demonstrates that acute symptomatic ICAO can be differentiated from chronic asymptomatic ICAO based on perfusion characteristics. Acute symptomatic ICAO can be precisely identified by the presence of a Tmax >10 s lesion exceeding 2 mL or prolonged MTT on a qualitative perfusion map review. Our decision algorithm displays strong agreement for potential clinical use. Validation in other ICAO cohorts is necessary.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.02768.
Test characteristics for predicting an acute symptomatic internal carotid artery occlusion based on perfusion patterns
Flowchart of the study design. ICAO, internal carotid artery occlusion; CGMH, Chang Gung Memorial Hospital; MRI, magnetic resonance imaging; CBV, cerebral blood volume; CBF, cerebral blood flow; MTT, mean transit time; HIR, hypoperfusion intensity ratio; DEFUSE 2, Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2; DEFUSE 3, Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3. *Patients were excluded due to >50% stenosis at contralateral internal carotid artery/middle cerebral artery or poor quality of images.
Qualitative assessment of perfusion maps from patients with asymptomatic and symptomatic internal carotid artery occlusion (ICAO). A total of 38 asymptomatic and 48 symptomatic ICAO cases were rated. For each map, the affected hemisphere was rated as showing a lesion that was “increased,” “symmetrical” (i.e., no apparent lesion), or “decreased” compared to the other hemisphere. If the affected hemisphere showed regions of decreased signal as well as regions of increased signal, the scan was rated as “heterogeneous.” This finding was only noted on the CBV map in a minority (19%) of the patients with symptomatic ICAOs. MTT, mean transit time; CBF, cerebral blood flow; CBV, cerebral blood volume.
Magnetic resonance imaging perfusion maps of patients with symptomatic and asymptomatic internal carotid artery occlusions (ICAO). Perfusion parameters (from right to left) include Tmax, mean transit time (MTT), cerebral blood flow (CBF), and cerebral blood volume (CBV). Typical perfusion profiles of (A) a patient with an acute symptomatic left ICAO, showing increased Tmax, increased MTT, decreased CBF, and decreased CBV in the left hemisphere and (B) a patient with a chronic asymptomatic right ICAO, showing slightly delayed Tmax (light blue), symmetrical MTT, symmetrical CBF, and symmetrical CBV in the right hemisphere.
Notes
Funding statement
Ting-Yu Chang received the funding support form Chang Gung Memorial Hospital by grant CMRPG3K1661.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: TYC, SC, MGL. Study design: TYC, SC, MGL. Methodology: TYC, SC, MM, MGL. Data collection: TYC, JJH, MPM, SK, GWA, THL, MGL. Investigation: TYC, SC, JJH, SL, MEM, LVO, GWA, AS, MGL. Statistical analysis: MM. Writing—original draft: TYC, MGL. Writing—review & editing: TYC, GWA, AS, MGL. Funding acquisition: TYC, GWA, MGL. Approval of final manuscript: all authors.
Acknowledgements
The contributions to the data presented in the study are all included in the article and supplementary material. More related study material is available at: http://dx.doi.org/10.17632/sbyhprjx3h.1 or https://data.mendeley.com/datasets/sbyhprjx3h/1.