Standard Versus Intensive Blood Pressure Control in Acute Ischemic Stroke Patients Successfully Treated With Endovascular Thrombectomy: A Systemic Review and Meta-Analysis of Randomized Controlled Trials
Article information
Abstract
Background and Purpose
The optimal blood pressure (BP) control after successful endovascular thrombectomy (EVT) in acute ischemic stroke (AIS) with large vessel occlusion (LVO) remains debatable. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) that evaluate the efficacy and safety of standard BP control (with systolic BP ≤180 mm Hg) versus intensive BP control (systolic BP <140 mm Hg) during the 24 hours after successful EVT in AIS with LVO.
Methods
PubMed, Scopus, the Cochrane Central Register of Controlled Trials, and Embase were searched to identify relevant trials. The crude odds ratio (OR) and 95% confidence interval (CI) were calculated and estimates using random-effects models were pooled. This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PROSPERO ID: CRD42023450673).
Results
Four RCTs involving 1,559 participants were included. Regarding efficacy outcomes, intensive BP control was associated with a lower likelihood of functional independence (OR: 0.68; 95% CI: 0.51–0.91 for modified Rankin Scale [mRS] ≤2) and walking without assistance (OR: 0.65; 95% CI: 0.53–0.81 for mRS ≤3). For safety outcomes, consistent with the efficacy findings, intensive BP control was significantly associated with severe disability or death (mRS 5 or 6) (OR: 1.34; 95% CI: 1.07–1.69). However, there were no significant differences including all-cause mortality, any intracerebral hemorrhage (ICH), symptomatic ICH, parenchymal hematoma type 2, and stroke recurrence.
Conclusion
While all four RCTs were conducted to demonstrate the superiority of intensive BP control over standard BP control, standard BP control may be beneficial for the outcome after EVT for AIS with LVO without increasing adverse safety outcomes. Caution should be needed with the application of intensive BP control during the 24 hours following successful recanalization after EVT.
Introduction
Endovascular thrombectomy (EVT) has been established as an effective therapy for acute ischemic stroke (AIS) with large vessel occlusion (LVO) [1,2]. Despite successful recanalization after EVT, a significant number of patients do not achieve functional independence [3-5]. One of the factors associated with such discrepancies could be blood pressure (BP) that influences the post-procedural perfusion status of the brain. For example, persistent elevation of BP after EVT could lead to poor neurological outcomes, increasing the risk of intracerebral hemorrhage (ICH) and cerebral edema [6-8]. Conversely, if the microvascular networks in the brain are compromised due to ischemia, they may become more susceptible to changes in BP [9,10]. Under these circumstances, maintaining BP at a low target could potentially disrupt the delicate balance of blood flow, potentially exacerbating damage to the ischemic brain tissue [11].
Current stroke guidelines recommend a target systolic BP (SBP) of less than 180 mm Hg and diastolic BP of less than 105 mm Hg in patients who have received EVT, for the 24 hours following the procedure [12,13]. However, these recommendations have been derived from earlier clinical trials on intravenous thrombolysis using tissue plasminogen activator, which may not be directly applicable to EVT [12,14]. Furthermore, these guidelines do not provide specific details on the optimal level below 180 mm Hg. Moreover, there have been discrepancies in the results of observational studies and clinical trials regarding BP levels after successful reperfusion of EVT [15-18]. In a previous observational study, an SBP level of ≥160 mm Hg was associated with a lower probability of achieving functional independence and a higher incidence of symptomatic ICH compared to a reference range of an SBP level of 100 mm Hg to 119 mm Hg in patients who had successful recanalization of EVT [16]. In addition, a recent large sample size meta-analysis of individual patients’ data from observational cohort studies demonstrated that increasing mean systolic BP levels in the first 24 hours after EVT were significantly associated with poor functional outcome, mortality, and early neurologic deterioration [19]. In contrast, a recent clinical trial demonstrated that intensive SBP targets (less than either 140 mm Hg or 160 mm Hg) during the 24 hours after successful EVT did not improve outcome compared with the standard SBP target (180 mm Hg or less) [20]. Accordingly, the evidence regarding the most effective and safe threshold of SBP after EVT has been inconsistent and inconclusive.
Therefore, establishing the optimal BP target after EVT is essential to provide guidance for healthcare professionals to achieve the best functional outcome after successful EVT. Thus, we conducted a systematic review and meta-analysis of all relevant randomized controlled trials (RCTs) that evaluate the efficacy and safety of standard BP control (SBP ≤180 mm Hg) versus intensive BP control (SBP <140 mm Hg) during the 24 hours after successful EVT in AIS with LVO.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines and the methodology in the Cochrane Handbook for Systematic Reviews of Interventions to extract data and assess the validity of this meta-analysis (Appendix 1) [21,22]. The included studies obtained approval from their institutional review boards or ethics committees for their protocols. Additionally, all participants reportedly either provided written or verbally confirmed their informed consent. Our protocol was registered with PROSPERO (CRD42023450673).
Search strategy and eligibility criteria
Two investigators (H.P. and S.I.S.) independently screened and discrepancies were resolved by discussion with a third investigator (T.J.S.). We searched PubMed (MEDLINE), Scopus, the Cochrane Central Register of Controlled Trials, and Embase, from inception to October 15, 2023. We restricted the search to studies on humans and RCTs without any language restrictions. The search strategies are shown in Supplementary Table 1. Criteria for inclusion of a study in our meta-analysis were as follows: (1) the study design was an RCT; (2) EVT in AIS with LVO; (3) the study evaluated intensive BP control versus standard BP control during the 24 hours after successful EVT in AIS with LVO; and (4) the study compared extensive outcome parameters between intensive and standard control.
Study selection and data extraction
The results retrieved from all databases were organized in a structured format, and any duplicate entries were removed. The remaining results were independently reviewed by three authors (G.H.L., M.K., and Y.H.K.), and any discrepant judgments were resolved through joint discussion. The screening process consisted of two distinct stages: first, a review of titles and abstracts to evaluate each study’s relevance for inclusion in our meta-analysis, and second, a comprehensive assessment of the fulltext documents according to predefined eligibility criteria to ascertain their suitability for inclusion in both qualitative and quantitative analyses. The extracted data from the included RCTs were as follows: (1) summary of included studies, including study name, publication year, first author’s name, country conducting the study, presence of blinding, inclusion criteria, total number of participants, the definition of intensive and standard BP control, and follow-up duration; (2) baseline characteristics of participants in each study, including age, sex, initial SBP and diastolic BP, baseline National Institutes of Health Stroke Scale (NIHSS) score, prior medication history before admission, and previous medical history; (3) study outcomes, including NIHSS, modified Rankin Scale (mRS); and (4) imaging findings, including hemorrhagic transformation after EVT.
Outcomes
The efficacy outcomes of our study were functional independence defined as mRS ≤2, minimal or no disability (mRS≤1), walking without assistance (mRS≤3), and NIHSS change at 24 hours. Safety outcomes included severe disability or death (mRS 5 or 6), all-cause mortality at 3 months, any ICH, symptomatic ICH, parenchymal hematoma type 2 (PH2) according to the European Cooperative Acute Stroke Study III (ECASS-III) criteria [23], and stroke recurrence during a 3-month follow-up.
Quality assessment
The quality of each included study was evaluated using the revised Cochrane Risk of Bias tool (ROB 2.0) across five bias domains [24]. The domains under scrutiny, which focused on evaluating potential biases originating from the randomization process, are bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result. Two independent researchers (H.P. and S.I.S.) assessed each criterion and discussed discrepancies until consensus was reached. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines by two reviewers (H.P. and T.J.S.) [25]. The GRADE guidelines, which consist of inconsistency, imprecision, indirectness, publication bias, and risk of bias, were evaluated for each outcome. Any discrepancies were resolved through discussion between the two reviewers. Publication bias can be assessed when the number of included studies is at least 10.
Statistical analysis
Outcomes were presented in terms of odds ratios (ORs) and 95% confidence intervals (CIs). To evaluate the presence and extent of heterogeneity among the included studies, the chi-square test was used to determine the presence of heterogeneity, and the I-square test was used to quantify the degree of heterogeneity. I-square percentages of 25%, 50%, and 75% were regarded as indicative of low, moderate, and high heterogeneity, respectively. If heterogeneity surpassed 50%, we applied the random-effects model; otherwise, we used the fixed-effects model. The Cochrane Collaboration’s Review Manager (RevMan, version 5.3; London, UK) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for meta-analysis, and GRADEpro GDT (GRADEpro Guideline Development Tool, McMaster University and Evidence Prime; www.gradepro.org) was used to assess the certainty of evidence for this meta-analysis.
Results
Study characteristics
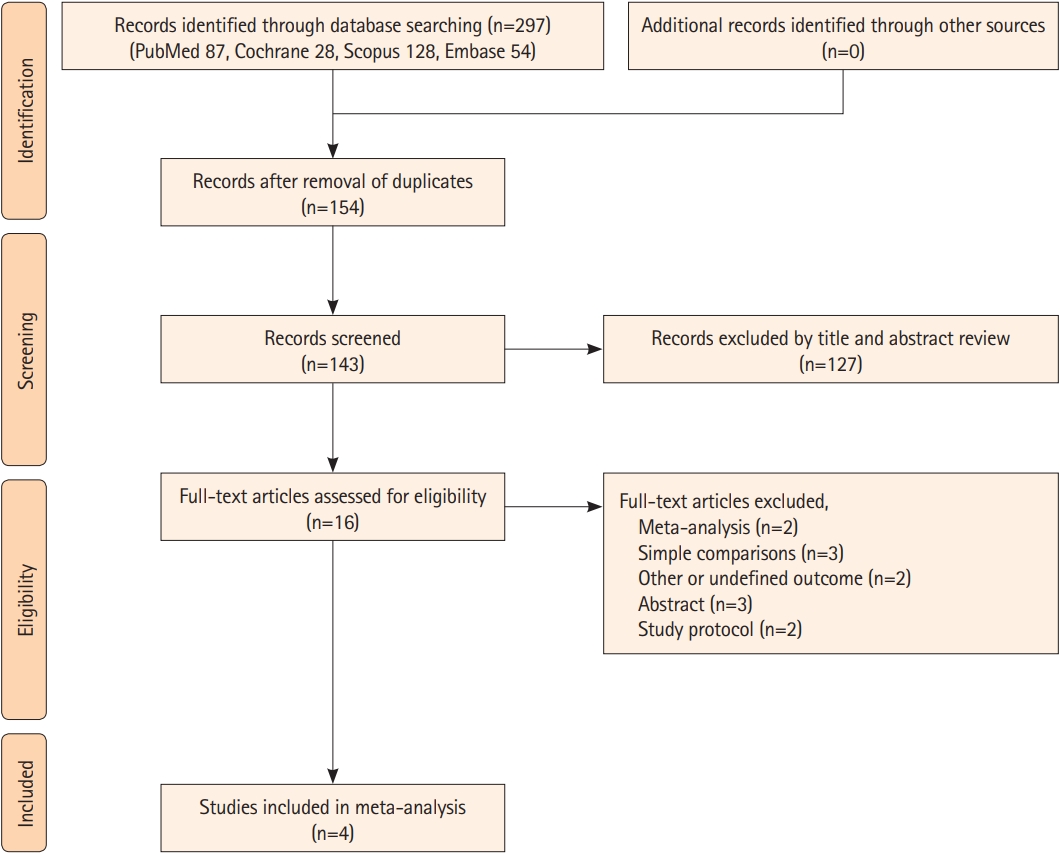
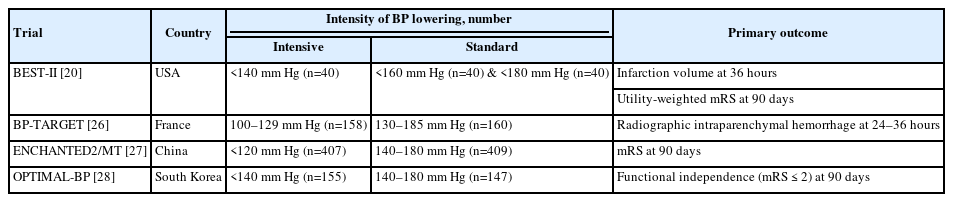
The final analysis included four RCTs including 1,559 participants (Figure 1) [20,26-28]. Two studies were conducted in Asian countries (ENCAHNTED2/MT [27] in China and OPTIMAL-BP [28] in South Korea), and the other two were conducted in the United States (BEST-II) [20] and France (BP-TARGET) [26]. The study design of three studies (ENCHANTED2/MT, OPTIMAL-BP, BEST-II) was a multicenter, blinded end-point study, and the endpoint was not blinded in one study (BP-TARGET) because they collected the primary outcome between 24 and 36 hours from EVT before the 3-month follow-up. All four RCTs were open-label, and the primary outcome was functional independence at 3 months. The summarization and baseline characteristics of the included four RCTs are shown in Tables 1 and 2. Mean SBP differences between intensive and standard BP control in four RCTs was 11.25 mm Hg (Supplementary Table 2). The overall proportion of time spent in the target range was 61.0% but this percentage did not include data of the ENCHANTED2/MT trial because the trial did not provide specific data regarding the proportion of time spent in the target range (Supplementary Table 3). Among four RCTs, one trial (BEST-II) categorized patients into three groups based on SBP targets: (1) SBP <140 mm Hg, (2) SBP <160 mm Hg, and (3) SBP <180 mm Hg without setting a definition of intensive BP control [20]. Considering the definition of intensive BP control in other three RCTs [26-28], the group managed with SBP <140 mm Hg in BEST-II trial was classified as intensive BP control to increase homogeneity of the intensive control group in our meta-analysis. Furthermore, additional sensitivity analysis in which we classified the group controlled with SBP <160 mm Hg from the BEST-II trial as intensive BP control was performed because the reference group in the BEST-II trial was consisted of those controlled with SBP <140 mm Hg and <160 mm Hg.
Risk of bias assessment and certainty of evidence
All studies included in our meta-analysis had a low risk of bias across five domains. The Cochrane risk-of-bias assessment for the included studies is summarized in Supplementary Figures 1 and 2. In addition, the certainty of evidence using the GRADE guidelines for each outcome is outlined in Supplementary Table 4.
Efficacy outcomes
The intensive BP control was significantly associated with a lower likelihood of achieving favorable functional independence (OR, 0.68; 95% CI, 0.51–0.91, P=0.009) (Figure 2A) and walking without assistance (mRS≤3) (OR, 0.65; 95% CI, 0.53–0.81, P<0.001) (Figure 2B). The overall certainty of evidence in GRADE guidelines about favorable functional independence and walking without assistance was moderate (Supplementary Table 4). However, there was no significant difference between the intensive BP and standard BP control regarding minimal or no disability (mRS ≤1) (OR, 0.82; 95% CI, 0.63–1.06, P=0.177) (Figure 2C). Additionally, NIHSS changes at 24 hours also showed a better trend toward the standard BP control without significance (mean difference, 0.43; 95% CI, -0.01–0.87, P=0.054) (Figure 2D). Pooled studies were homogeneous in functional independence (I2=39%), walking without assistance (I2=0%), minimal or no disability (I2=25%), and NIHSS change (I2=11%). In sensitivity analysis with SBP <160 mm Hg group as intensive BP control from the BEST-II trial, in line with primary analysis, the intensive BP control showed a significantly lower likelihood of achieving functional independence and walking without assistance (Supplementary Figure 3).
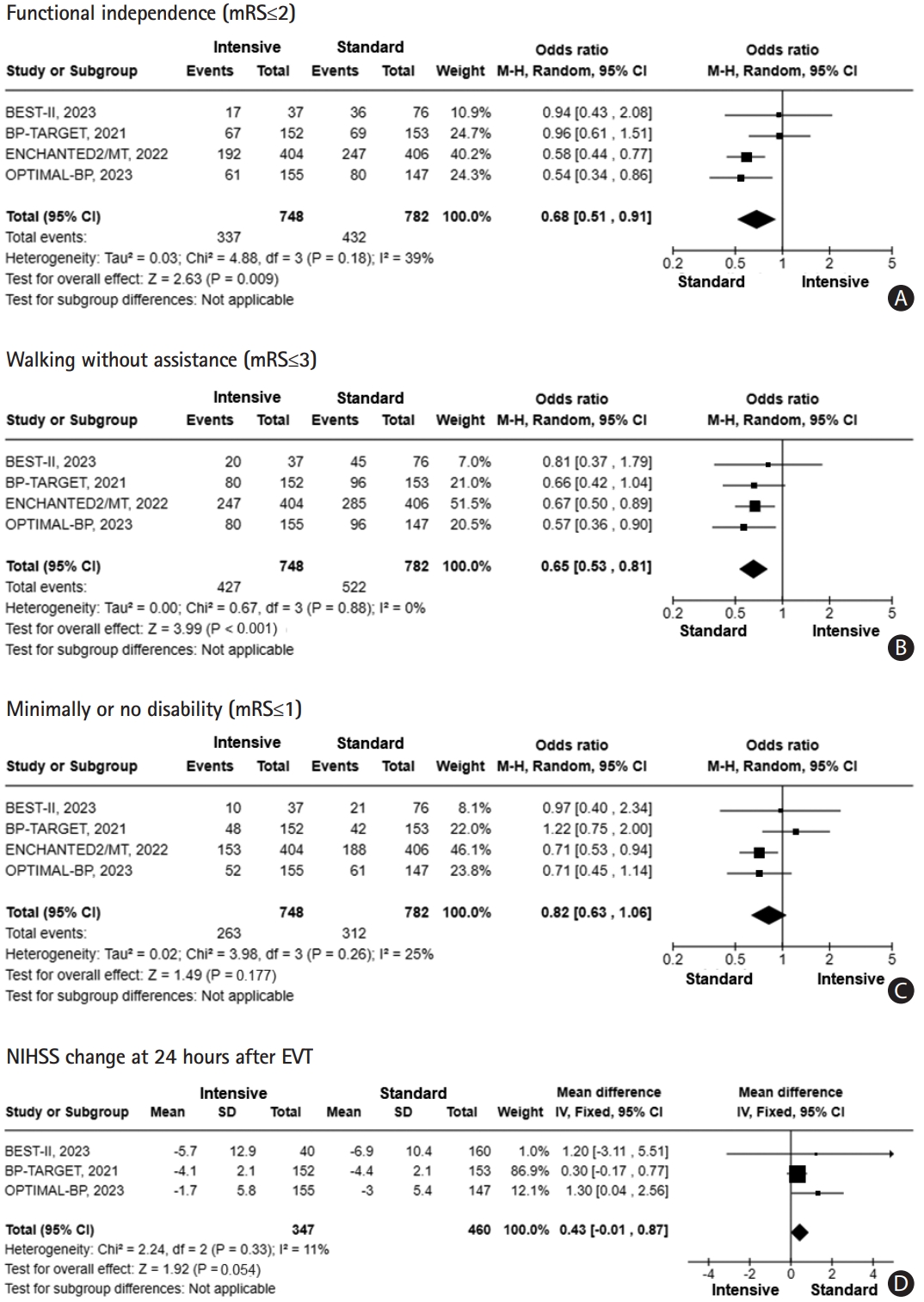
Forest plot of the efficacy outcomes [20,26-28]. (A) Functional independence (mRS≤2). (B) Walking without assistance (mRS≤3). (C) Minimally or no disability (mRS ≤1). (D) NIHSS change at 24 hours after endovascular thrombectomy. mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; EVT, endovascular thrombectomy; CI, confidence interval.
Safety outcomes
The intensive BP control showed a significantly higher probability of having severe disability or death (mRS 5 or 6) (OR, 1.34; 95% CI, 1.07–1.69, P=0.013) (Figure 3A). The overall certainty of evidence in GRADE guidelines about this finding was moderate (Supplementary Table 4). Otherwise, there was no significant difference in all-cause mortality (OR, 1.15; 95% CI, 0.87–1.53, P=0.330) (Figure 3B), any ICH (OR, 1.09; 95% CI, 0.88–1.35, P=0.455) (Figure 3C), symptomatic ICH (OR, 1.19; 95% CI, 0.80–1.76, P=0.401) (Figure 3D), PH2 (OR, 1.00; 95% CI, 0.60–1.66, P=0.924) (Figure 3E), and stroke recurrence (OR, 1.20; 95% CI, 0.67–2.17, P=0.518) (Figure 3F). In terms of heterogeneity, there was no significant heterogeneity (I2=3% in severe disability or death, I2=0% in all-cause mortality, any ICH, symptomatic ICH, PH2, and stroke recurrence) in severe disability or death, all-cause mortality at 3 months, any ICH, symptomatic ICH, PH2, and stroke recurrence. In sensitivity analysis with SBP <160 mm Hg group as intensive BP control from the BEST-II trial, intensive BP control had a higher probability of having significant disability or death (mRS 5 or 6) (Supplementary Figure 4).

Forest plot of the safety outcomes [20,26-28]. (A) Severe disability or death (mRS 5 or 6). (B) All-cause mortality. (C) Any intracerebral hemorrhage. (D) Symptomatic intracerebral hemorrhage. mRS, modified Rankin Scale; ICH, intracerebral hemorrhage; CI, confidence interval. (E) Parenchymal hematoma type 2 (PH2). (F) Stroke recurrence. CI, confidence interval.
Discussion
In this meta-analysis using four RCTs about BP control after successful EVT, we found that intensive BP control was associated with a reduced probability of functional independence (mRS≤2) and walking without assistance (mRS≤3). Additionally, consistent with the direction of functional independence and walking without assistance, the likelihood of severe disability or death (mRS 5 or 6) was significantly increased in intensive BP control. In safety outcomes, there was no significant difference in all-cause mortality, any ICH, symptomatic ICH, PH2, and stroke recurrence. In addition, in sensitivity analysis, consistent to initial results, the intensive BP control showed a significantly lower likelihood of achieving functional independence and walking without assistance, while the standard BP control had a lower probability of significant disability or death.
Managing BP can be especially challenging for patients with AIS when considering that this type of stroke arises from the interruption of blood flow to a specific area of the brain, which is influenced by BP. High BP is a risk factor for the development of AIS and other cardiovascular events [29,30], but in our study, significantly lowering BP immediately in AIS is not always recommended, particularly in patients with successful recanalization after EVT. The brain has a mechanism known as cerebral autoregulation that ensures consistent cerebral blood flow despite changes in SBP [31]. Following AIS, this autoregulation may be disrupted, rendering the brain more susceptible to changes in BP. Accordingly, a substantial reduction in SBP after successful recanalization after EVT can potentially reduce cerebral perfusion pressure, possibly leading to additional damage in brain tissue already vulnerable [32].
In particular, patients with LVO who received EVT may already be hemodynamically unstable, as these individuals often present with a moderate to large infarct core or reperfusion-related injury before recanalization [33,34]. Therefore, rapidly lowering BP can exacerbate this instability, potentially leading to further organ dysfunction [35]. The ischemic penumbra is at risk of permanent damage but may still be viable for a certain period. Before recanalization, this ischemic penumbra may depend on higher BP to maintain cerebral perfusion pressure due to the loss of cerebral autoregulation. Under these circumstances, excessively reducing BP after successful recanalization may extend the damage to this ischemic penumbra [32].
In previous observational studies, elevated BP was associated with a higher likelihood of poor outcome and risk of ICH [15-19]. These results have a discrepancy with our meta-analysis using RCTs. This discrepancy may be originated from study design such as various uncontrolled confounding factors, the lack of data regarding how successfully SBP was controlled among the different groups during monitoring period, and absence of standardized BP measurement protocol in observational studies.
In our study, the rates of any ICH, symptomatic ICH, and severe hemorrhagic transformation (PH2) were similar between the two groups. The four RCTs that were included set the maximal target SBP to be below 180 mm Hg based on current guidelines. Thus, in accordance with current guidelines [12,13], when SBP is controlled under 180 mm Hg, the incidence of ICH was not different between intensive and standard BP control.
Moreover, elevated SBP is well-known to be a risk factor for hemorrhagic transformation after EVT [36,37], but detailed SBP targets to mitigate ICH or severe hemorrhagic transformation after EVT have not been clearly proposed [36]. Although RCT-based evidence is still lacking regarding the development of symptomatic ICH or hemorrhagic transformation after EVT when SBP is adjusted above 180 mm Hg, our meta-analysis is meaningful in showing that an SBP 180 mm Hg or less may not increase the risk of developing critical issues such as symptomatic ICH or severe hemorrhagic transformation.
In four RCTs, the standard BP control was defined as having an SBP <180–185 mm Hg. However, the mean SBP throughout the 24-hour period in the standard BP control group was consistently maintained within the range of 140–160 mm Hg [20,26-28]. Thus, there was a relatively small mean SBP difference between the standard and intensive BP control groups. In addition, a substantial portion of patients did not stay within the specified BP target of each group during the 24-hour monitoring period. Therefore, in the future, additional trials should be conducted, using different BP targets, and for patients who have successfully maintained their BP within the target range.
Our study had several limitations. First, although our intensive SBP control group was based on an SBP less than 140 mm Hg, there were two clinical trials (BP-TARGET and ENCHANTED2/MT) that defined the intensive arm as SBP 100–129 mm Hg (BP-TARGET) or <120 mm Hg (ENCHANTED2/MT) [26,27]. Thus, there is an issue with the intensive BP control group being heterogeneous in our study. Pooled analysis using individual patient data from four RCTs should be conducted in the future. Second, analysis of baseline characteristics for all participants and subgroup analysis based on stroke subtype and underlying vascular status was not possible due to the absence of individual data from the RCTs included in our meta-analysis. Third, because of the difficulties with conducting double-blinded studies in the context of clinical trials regarding BP control in AIS with LVO after successful recanalization, the significance of the findings in our study may be relatively limited.
Conclusions
Our meta-analysis showed that intensive SBP control during the 24 hours after successful recanalization following EVT was associated with a higher risk of poor neurological outcomes compared to standard SBP control. Therefore, caution is warranted, as the strategy of aggressively lowering SBP during the 24 hours following successful recanalization after EVT may lead to a poorer prognosis compared to the standard BP control.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.04119.
Keywords used in searching strategies
Mean SBP differences of each trial
Proportion of time spent in the target in each trial
GRADE guidelines about each outcome
The risk of bias summary in included studies.
The risk of bias graph in included studies.
Forest plot of the efficacy outcome as sensitivity analysis [20,26-28]. (A) Functional independence (mRS≤2). (B) Walking without assistance (mRS≤3). (C) Minimally or no disability (mRS≤1). (D) NIHSS change at 24 hours after endovascular thrombectomy. mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; EVT, endovascular thrombectomy; CI, confidence interval.
Forest plot of the safety outcomes as sensitivity analysis [20,26-28]. (A) Severe disability or death (mRS 5 or 6). (B) All-cause mortality. (C) Any intracerebral hemorrhage. (D) Symptomatic intracerebral hemorrhage. mRS, modified Rankin Scale; ICH, intracerebral hemorrhage; CI, confidence interval.
Notes
Funding statement
This work was supported by an Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korean government (MSIT) (2022–0-00621 to TJS, Development of artificial intelligence technology that provides dialog-based multi-modal explainability). This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-00262087 to TJS). The funding source had no role in the design, conduct, or reporting of this study.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: HP, TJS. Study design: HP, TJS. Methodology: HP, SIS, TJS. Data collection: GHL, MK, YHK. Statistical analysis: HP. Writing—original draft: HP, TJS. Writing—review & editing: TJS. Funding acquisition: TJS. Approval of final manuscript: all authors.