Haptoglobin Gene Polymorphism Is Associated With Lower Postoperative Cardiovascular Risk in Carotid Stenosis Patients
Article information
Dear Sir:
The human haptoglobin (Hp) gene locus is polymorphic, containing two prevalent alleles: Hp1 and Hp2. Further differentiation of the Hp alleles arises from nucleotide polymorphisms and copy number variations, leading to subtypes such as Hp1F, Hp1S, Hp2FS, and Hp2SS where “F” and “S” here denote the fast and slow protein gel migration rates, respectively [1]. Studies have shown that the Hp1 allele is associated with low cholesterol levels in a large population study (n=22,288) [2]. The Hp1 allele has also been associated with a lower risk of suffering from symptomatic carotid stenosis, atherosclerosis [3], and major cardiovascular diseases including both ischemic stoke and ischemic heart disease in a cross-sectional study of patients with carotid stenosis (n<100) [4]. However, the associations between Hp alleles and cardiovascular diseases are not observed in specific populations such as patients with type 1 diabetes [5] or subjects with glycosylated hemoglobin A1c (HbA1c) <6.5% [6], suggesting that the effects of Hp alleles in atherosclerosis remain to be fully understood. To date, no studies have examined the distinct roles of Hp subtypes in atherosclerosis or their impact on cardiovascular events (CVE).
To address this, we investigated the associations between Hp, Hp subtypes and atherosclerosis as well as CVE using the Carotid Plaque Imaging Project (CPIP) biobank (Lund University, Malmö, Sweden). Data was utilized from 395 carotid endarterectomy patients. Genotyping was carried out using the Illumina Infinium OmniExpress-24 BeadChip array (San Diego, CA, USA), and Hp gene polymorphisms were subsequently imputed. For histopathological analyses, we randomly selected 45 carotid plaques. Patients were followed longitudinally for CVE. A comprehensive methods section is available in the Supplementary Methods.
The baseline clinical characteristics of the CPIP cohort are presented in Supplementary Table 1. No significant differences at baseline were identified when comparing Hp1S carriers (n=141, 35.6%) and non-Hp1S carriers (n=254, 64.4%), nor in the sub-group of subjects used for histological analyses (Supplementary Table 2). Hp subtypes were found to be in Hardy-Weinberg equilibrium (Supplementary Tables 3-6).
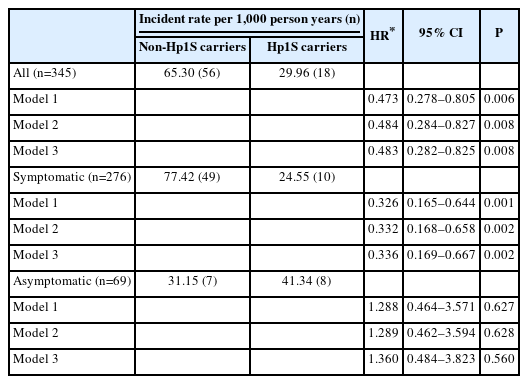
During a median follow-up of 51 months (interquartile range 26–93), 85 subjects developed postoperative CVE. Among patients carring Hp2 and Hp subtypes Hp1F, Hp2SS, and Hp2FS, Hp1S carriers demonstrated a lower risk of postoperative CVE, especially among symptomatic patients (Figure 1A and Supplementary Figure 1). After accounting for potential confounding factors such as age, sex, and low-density lipoprotein (LDL) cholesterol levels, Hp1S was associated with a lower risk of suffering from postoperative CVE particularly in symptomatic patients (hazard ratio=0.336, 95% confidence interval 0.169–0.667, P=0.002) (Table 1). We did not observe a significant association between Hp1S and postoperative CVE among asymptomatic patients, nor a significant interaction between Hp1S and the presence of preoperative cerebrovascular symptoms.
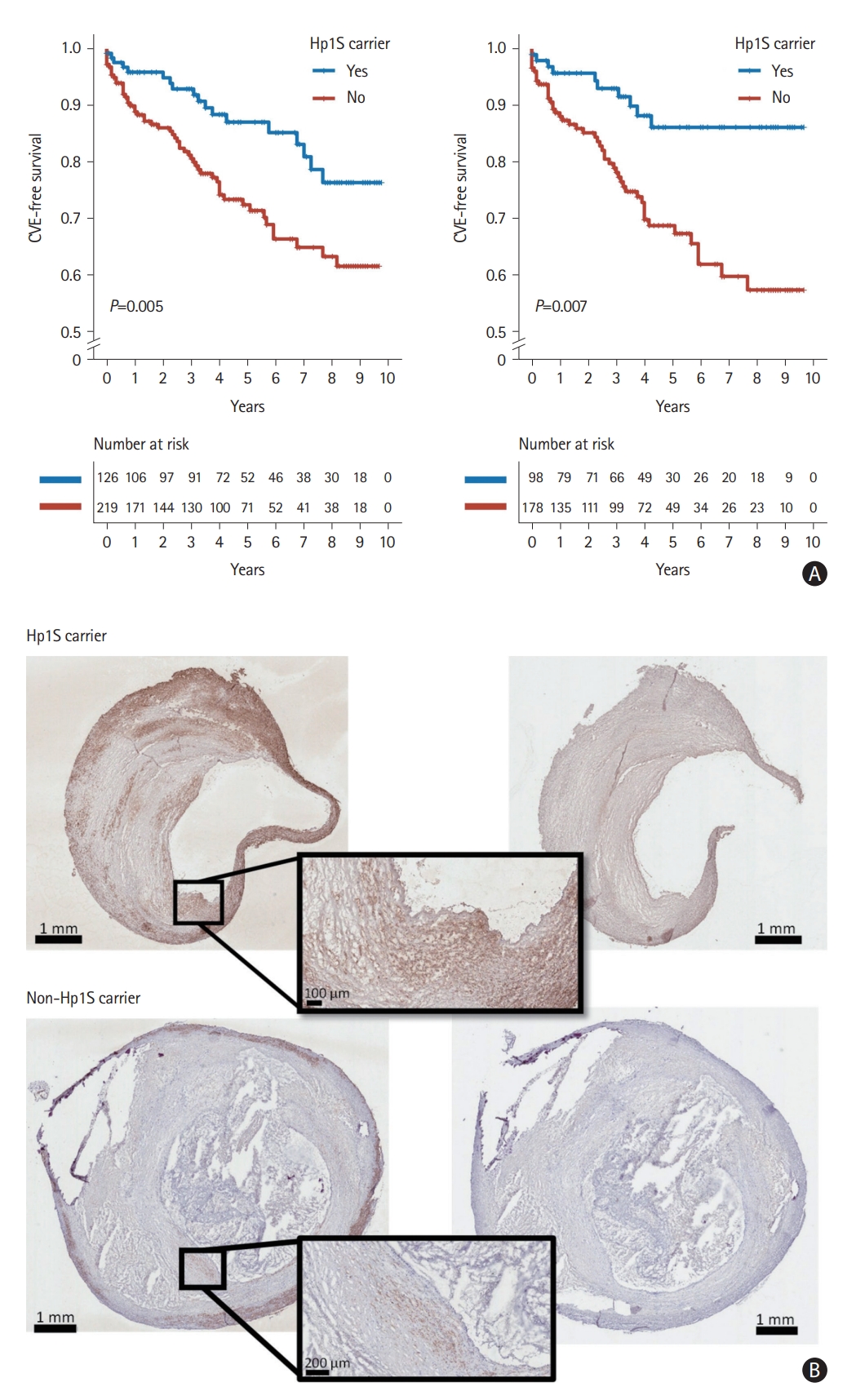
The association between Hp1S and atherosclerosis. (A) Kaplan-Meier curves depicting the time to cardiovascular events (CVE) for Hp1S carriers and non-carriers in the entire cohort (left) and among symptomatic patients (right). (B) Representative human atherosclerotic plaque tissue section images showing a greater plaque area stained positive for the smooth muscle cell marker alpha-actin (in brown) in plaques from Hp1S carrier compared to plaques from non-carrier patients. Positively stained plaque tissue sections on the left and isotype-control tissue sections on the right.
Carotid plaques from Hp1S carriers had a significantly greater plaque area stained positive for the smooth muscle cell (SMC) marker alpha-actin (5.0% [3.7%–7.5%] vs. 3.6% [1.9%–5.2%], Mann-Whitney U test: P=0.033; permutation test: P=0.024) than plaques from non-carriers (Figure 1B and Supplementary Figure 2), but no other of the measured histological components (Oil Red O, glycophorin A, CD68, and Movat pentachrome) were significantly different between plaques from Hp1S carriers and non-carriers (Supplementary Table 7).
The underlying mechanism of Hp in atherosclerosis remains unclear. One possible explanation is that Hp2 may have a lower antioxidative capacity compared to Hp1, as suggested by previous research [7]. This difference is also reflected in lower levels of LDL and total cholesterol in carriers of Hp1 [2]. Another study later found that the risk of coronary heart disease was more pronounced in Hp2-2 carriers with hyperglycemia, suggesting that glycosylation of hemoglobin might impair Hp2’s ability to act as an antioxidant [6]. Further, intensive glucose-lowering therapy demonstrated the potential to prevent coronary heart disease [8]. However, it is important to note that Hp genotypes were not associated with stroke in type 1 diabetes or in patients with elevated HbA1c levels. Notably, these studies primarily focused on the differences between the main Hp genotype, whereas the subtypes of Hp1F, Hp1S, Hp2FS, and Hp2SS have not been examined [2,9]. For the first time, our findings reveal differences between haptoglobin subtypes in atherosclerosis. These findings may help to shed light on the inconsistencies in cardiovascular risk stratification based solely on Hp1 or Hp2 alleles.
In summary, we found that symptomatic patients carrying Hp1S had a lower risk of developing postoperative CVE after carotid endarterectomy surgery. Plaques from Hp1S carriers demonstrated a greater plaque area of SMC, potentially contributing to plaque stability. These findings suggest a protective role of the Hp1S against postoperative CVE. Hp1 subtypes may be a valuable tool to improve risk stratification in symptomatic patients undergoing carotid endarterectomy, helping to identify patients that would benefit of particular intensive monitoring or secondary prevention.
Several limitations should be considered. Our results apply only to patients suffering from advanced carotid atherosclerosis and may not be extended to other populations. The small sample size in the immunohistochemical analysis may introduce bias, even though a permutation test suggests the difference is less likely to be the result of selection bias. Lastly, the precise biological role of Hp1S remains to be further investigated to fully understand the underlying mechanisms in atherosclerosis and CVE. Further investigation is necessary to fully understand the underlying mechanisms and to confirm the association between Hp1S and postoperative CVE.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.03349.
Baseline clinical characteristics in relations to Hp1S allele
Baseline clinical characteristics in relations to Hp1S allele for subjects with plaques assessed by immunohistochemistry
Hp genotypes are in Hardy-Weinberg Equilibrium in the CPIP cohort (n=395)
Hp phenotype frequency in the CPIP cohort (n=395)
Frequency of Hp subtypes (n=90) in subjects with plaques assessed by immunohistochemistry
Hp phenotype frequency in subjects with plaques assessed by immunohistochemistry (n=45)
Immunohistochemical results for Hp1S carriers and non-Hp1S carriers
Kaplan-Meier curves depicting the time to cardiovascular events (CVE) in the entire cohort. (A) Hp1F carriers vs. non-carriers. (B) Hp2SS carriers vs. non-carriers. (C) Hp2FS carriers vs. non-carriers. (D) Hp2 carriers vs. non-carriers. The P-value from the log-rank test is reported.
The plaque area stained positive for the smooth muscle cell marker, alpha-actin, was significantly greater in plaques from Hp1S carriers compared to plaques from non-carriers. Mann-Whitney U test was used. *P<0.05, statistically significant.
Flow chart showing the number of patients included in the study and the statistical analysis. CPIP, Carotid Plaque Imaging Project cohort.
Notes
Funding statement
This work was supported by grants from the Swedish Research Council, the Swedish Society for Medical Research, the Swedish Heart and Lung Foundation, the Bo & Kerstin Hjelt Diabetes Foundation, Swedish Stroke Association, Söderström König Foundation, Skåne University Hospital funds, and Lund University Diabetes Center (Swedish Research Council - Strategic Research Area Exodiab Dnr 2009-1039, Linnaeus grant Dnr 349-2006-23 and the Swedish Foundation for Strategic Research Dnr IRC15-006). The Medical Faculty at Lund University and Region Skåne are acknowledged for generous financial support.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: IG, JS. Study design: IG, JS. Methodology: IG, JS. Data collection: AE, AS. Investigation: AE, AS. Statistical analysis: JS, YM. Writing—original draft: YM. Writing—review & editing: YM, IG, AE, JS. Funding acquisition: IG, AE, JS. Approval of final manuscript: all authors.
Acknowledgements
The authors are grateful to Lena Sundius, Ana Persson, and Mihaela Nitulescu for generous technical support.