Effect of Intravenous Thrombolysis Prior to Mechanical Thrombectomy According to the Location of M1 Occlusion
Article information
Abstract
Background and Purpose
The additive effects of intravenous thrombolysis (IVT) before mechanical thrombectomy (MT) remain unclear. We aimed to investigate the efficacy and safety of IVT prior to MT depending on the location of M1 occlusion.
Methods
We reviewed the cases of patients who underwent MT for emergent large-vessel occlusion of the M1 segment. Baseline characteristics as well as clinical and periprocedural variables were compared according to the location of M1 occlusion (i.e., proximal and distal M1 occlusion). The main outcome was the achievement of functional independence (modified Rankin Scale score, 0–2) at 3 months after stroke. The main outcomes were compared between the proximal and distal groups based on the use of IVT before MT.
Results
Among 271 patients (proximal occlusion, 44.6%; distal occlusion, 55.4%), 33.9% (41/121) with proximal occlusion and 24.7% (37/150) with distal occlusion underwent IVT prior to MT. Large-artery atherosclerosis was more common in patients with proximal M1 occlusion; cardioembolism was more common in those with distal M1 occlusion. In patients with proximal M1 occlusion, there was no association between IVT before MT and functional independence. In contrast, there was a significant association between the use of IVT prior to MT (odds ratio=5.30, 95% confidence interval=1.56–18.05, P=0.007) and functional independence in patients with distal M1 occlusion.
Conclusion
IVT before MT was associated with improved functional outcomes in patients with M1 occlusion, especially in those with distal M1 occlusion but not in those with proximal M1 occlusion.
Introduction
With the development of neuroimaging and interventional devices for acute ischemic stroke, mechanical thrombectomy (MT) has become an effective mode of treatment for patients with emergent large-vessel occlusion (LVO) in the anterior circulation [1]. The use of intravenous thrombolysis (IVT) prior to MT may have some potential benefits by increasing the chance of reperfusion; however, randomized clinical trials and meta-analyses comparing the efficacy of direct MT and IVT before MT have shown conflicting results [2-6].
The M1 segment of the middle cerebral artery (MCA) is the most common site targeted by MT [7]. Previous studies have shown that the efficacy of IVT differed according to the size, permeability, and nature of the clot [8-10]. Moreover, the occlusion site may also affect the efficacy of IVT. A previous study did not provide evidence that occlusion location significantly affected the efficacy of IVT [11]; however, MCA occlusions showed differences in characteristics according to location (proximal vs. distal M1 segment) [12]. The efficacy of IVT alone differed according to the location of the anterior circulation LVO [13,14]. Therefore, the additive effect of IVT before MT may differ between proximal and distal M1 occlusions.
This study aimed to investigate the efficacy and safety of IVT before MT as compared with direct MT in patients with MCA occlusion according to the location of the occlusion (proximal vs. distal M1 segment). In addition, we identified the clinical and periprocedural factors associated with the efficacy of IVT before MT according to the location of the M1 occlusion.
Methods
Study population
We retrospectively reviewed the records of patients admitted to the stroke center of Asan Medical Center between January 2013 and August 2022. Our stroke center performed MT in patients with emergent LVO, which was confirmed by computed tomography (CT) or magnetic resonance (MR) angiography (i.e., internal carotid artery, M1 or M2 segment of the MCA, basilar artery, or vertebral artery) within 6 hours from the last normal time or within 24 hours after the last normal time with significant diffusion-perfusion mismatch. This study included adult patients (age ≥18 years) who underwent MT for emergent LVO. Of them, we excluded (1) patients whose LVO location was not isolated unilateral M1 occlusion, and (2) those who underwent elective MT. The ethics committee of Asan Medical Center approved this study (approval No. 2022-1676) and waived the need for informed consent due to its retrospective nature.
Clinical data collection
We obtained data on baseline demographics, vascular risk factors, and the use of tissue plasminogen activators (tPA). IVT was performed by administering alteplase at 0.9 mg/kg for eligible patients with acute ischemic stroke within 4.5 hours from last normal time. Initial stroke severity was evaluated using the National Institutes of Health Stroke Scale (NIHSS) score; the stroke mechanism was defined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.
We also collected data on periprocedural variables, including onset-to-puncture time (OTPT), puncture-to-recanalization time (PTRT), onset-to-recanalization time (OTRT), baseline computed tomography Alberta Stroke Program Early CT Score (CT ASPECTS), MT methods (i.e., contact aspiration, stent retriever, and rescue angioplasty), first-pass effect, and modified Thrombolysis in Cerebral Infarction (mTICI) grade. Successful recanalization was defined as an mTICI grade 2B or 3.
For the detection of hemorrhagic transformation (HT) after MT, gradient-echo imaging was routinely examined. The type of HT was classified according to the European Cooperative Acute Stroke Study classification [15]. Symptomatic HT was defined as cerebral hemorrhage that was temporally associated with neurological deterioration as documented on gradient-echo imaging [16]. The main outcome was the achievement of functional independence (modified Rankin Scale [mRS] score of 0–2) at 3 months after stroke occurrence.
Classification of proximal and distal M1 occlusion
The location of the M1 occlusion was divided into proximal and distal occlusions according to the results of initial CT or MR angiography. MCA length was measured from the bifurcation points of the internal carotid artery to the MCA. When the occluded MCA was successfully recanalized, the lengths of the (a) measurable ipsilateral MCA with occlusion before MT and (b) ipsilateral MCA after successful MT were measured on cerebral angiography (Supplementary Figure 1). When recanalization of the occluded MCA was unsuccessful, the lengths of the (a) measurable ipsilateral MCA with occlusion before MT and (b) the contralateral MCA were measured on cerebral angiography. The location of M1 occlusion was defined based on the following formula: (a/b)×100, where the resulting values of 50 or less were categorized as proximal M1 occlusion while those over 50 were categorized as distal M1 occlusion [12].
The location of the M1 occlusion was classified by a neurointerventionist (J.C.R.) and a stroke neurologist (B.J.K.). All classification processes were independently performed; all researchers were blinded to the clinical data.
Statistical analysis
We first compared the clinical and periprocedural factors according to the location of M1 occlusion. The significance of intergroup differences was assessed using the Student’s t-test, Mann–Whitney U test, and chi-squared test, as appropriate. Using a multivariate logistic regression model, we identified factors significantly associated with functional independence, including variables from the univariate logistic regression model. Furthermore, we calculated the P values for the interactions between the location of the M1 occlusion and IVT (i.e., use of tPA) to achieve functional independence. We also divided the patients according to the location of M1 occlusion and compared the clinical variables and periprocedural factors according to the use of tPA in each group. Finally, variables with significant associations (P<0.10) in the univariate logistic regression analyses were included in the multivariate logistic regression analyses for each group. P values <0.05 were considered statistically significant. Odds ratios (ORs) and 95% confidence intervals (CIs) were also determined. All analyses were performed using the R Software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study period, 764 patients underwent MT for emergent LVO at our center. Among them, we excluded 481 patients who did not have an isolated M1 occlusion and 12 who underwent elective MT. Finally, a total of 271 patients were included in this study (Supplementary Figure 2).
The mean age of the patients was 68.6±12.7 years; 142 (52.4%) were men. Moreover, 121 (44.6%) patients were classified as having proximal M1 occlusion, whereas 150 (55.4%) had distal M1 occlusion; of these, 41 (33.9%) patients with proximal occlusion and 37 (24.7%) with distal occlusion received IVT prior to MT.
Baseline characteristics according to the location of M1 occlusion
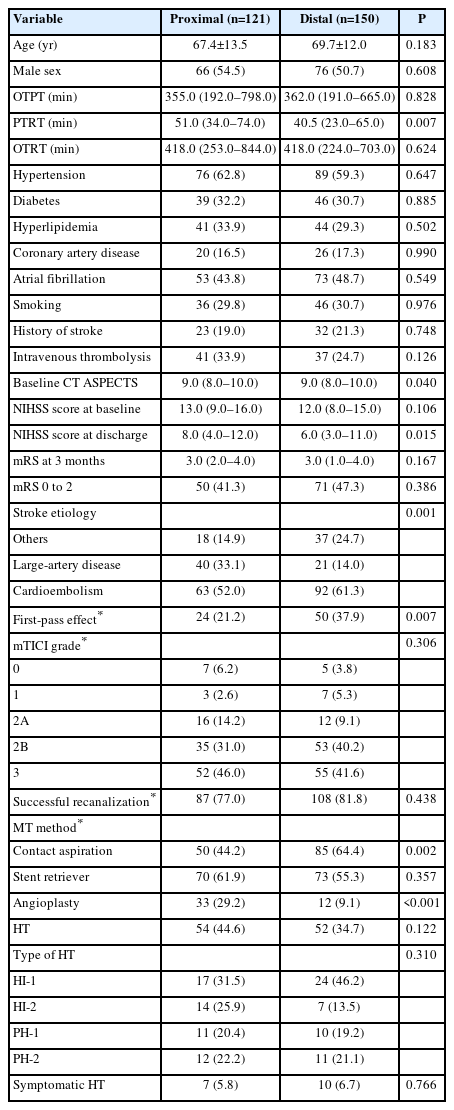
The clinical characteristics of the patients according to the location of the M1 occlusion are summarized in Table 1. There were no significant differences between clinical factors and functional independence at 3 months according to the location of the M1 occlusion. However, PTRT was longer in patients with proximal M1 occlusion than in those with distal M1 occlusion (51.0 [34.0–74.0] min vs. 40.5 [23.0–65.0] min, P=0.007). In addition, the stroke etiology was significantly different between the two groups (P=0.001), as the prevalence of large-artery atherosclerosis was higher in patients with proximal rather than with distal occlusion (40 [33.1%] vs. 21 [14.0%]); the prevalence of cardioembolism was lower in patients with proximal compared to those with distal occlusion (63 [52.0%] vs. 92 [61.3%]). Additionally, the proportion of first-pass effects was higher in patients with distal M1 occlusion (24 [21.2%] vs. 50 [37.9%], P=0.007). Contact aspiration was more common in patients with distal M1 occlusion (50 [44.2%] vs. 85 [64.4%], P=0.002), while angioplasty was more common in those with proximal M1 occlusion (33 [29.2%] vs. 12 [9.1%], P<0.001).
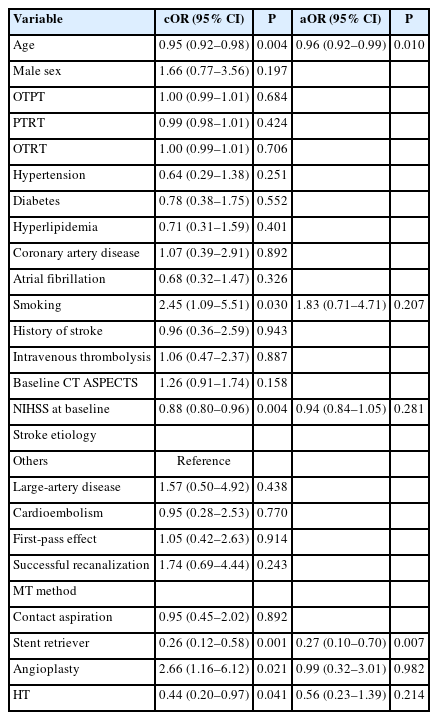
Univariate logistic regression analysis showed that functional independence (mRS score of 0–2) was significantly associated with age, hypertension, IVT before MT, baseline NIHSS score, successful recanalization, use of stent retrievers, and occurrence of HT. Multivariate analysis showed that age (OR 0.94 [95% CI, 0.91–0.96], P<0.001), IVT prior to MT (OR 2.64 [95% CI, 1.31–5.29], P=0.006), baseline NIHSS score (OR 0.93 [95% CI, 0.87–0.99], P=0.028), successful recanalization (OR 2.69 [95% CI, 1.22–5.93], P=0.014), use of stent retriever (OR 0.51 [95% CI, 0.27–0.96], P=0.036), and the occurrence of HT (OR 0.39 [95% CI, 0.21–0.72], P=0.003) were significantly associated with functional independence in patients with M1 occlusion (Supplementary Table 1). The P value for interaction between the location of M1 occlusion and IVT for functional independence was 0.005.
Effect of IVT prior to MT in patients with proximal M1 occlusion
Table 2 shows a comparison of the clinical and periprocedural factors according to the use of IVT before MT in patients with proximal M1 occlusion. Patients who received IVT before MT for proximal M1 occlusion had a shorter median OTPT (479.0 [311.5–967.5] min vs. 214.0 [160.0–300.0] min, P<0.001) and OTRT (530.0 [382.0–1,049.0] min vs. 275.0 [224.0–356.0] min, P<0.001) than those who did not receive IVT (Table 2). There were no significant differences between other factors, except for higher prevalence of cardioembolism in patients with IVT prior to MT than those without (35 [43.8%] vs. 28 [68.3%], P=0.019). Univariate logistic regression analysis showed that age, smoking, baseline NIHSS score, use of a stent retriever and angioplasty, and occurrence of HT were also associated with functional independence (Table 3). Multivariate analysis showed that age (adjusted OR 0.96 [95% CI, 0.92–0.99], P=0.010) and the use of stent retriever (adjusted OR 0.27 [95% CI, 0.10–0.70], P=0.007) were predictors for functional independence in patients with proximal M1 occlusion.
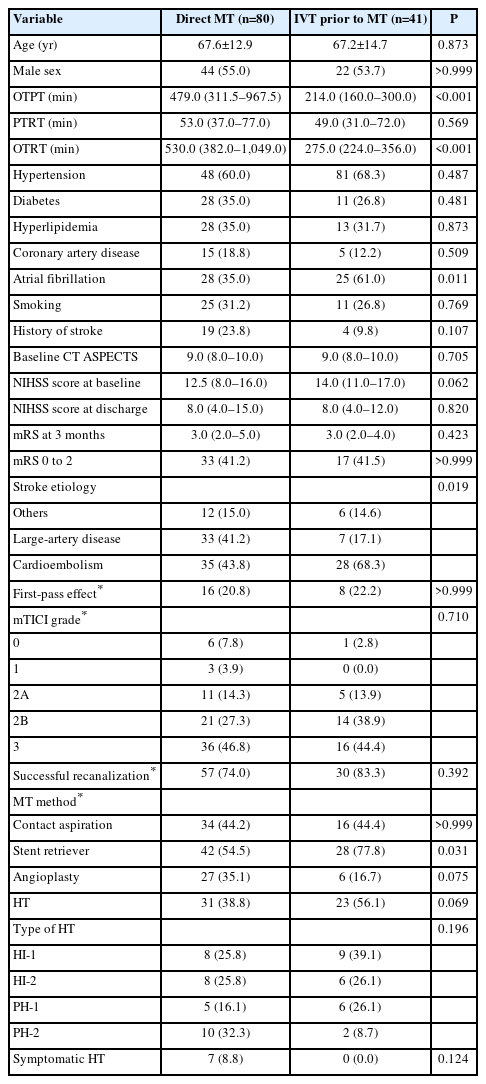
Comparison of clinical variables according to the use of IVT prior to MT in patients with proximal M1 occlusion
Effect of IVT prior to MT in patients with distal M1 occlusion
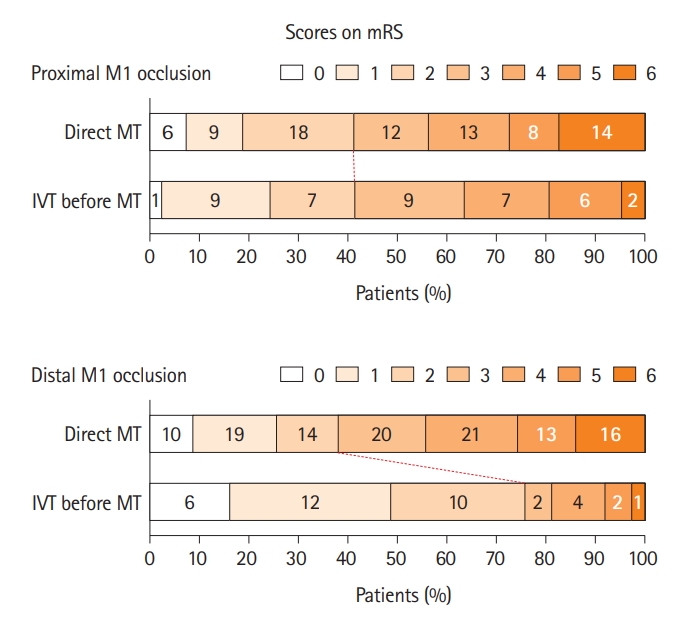
Table 4 shows a comparison of the clinical and periprocedural factors according to the use of IVT before MT in patients with distal M1 occlusion. Patients who received IVT before MT had a shorter median OTPT (505.0 [251.0–766.0] min vs. 189.0 [164.0–266.0] min, P<0.001) and OTRT (560.0 [292.0–836.0] min vs. 224.0 [184.0–309.0] min, P<0.001) than those who did not; atrial fibrillation was more common in those who received IVT before MT (49 [43.4%] vs. 24 [64.9%], P=0.037). Lastly, the functional outcome at 3 months was better in those who received IVT prior to MT (3.0 [1.0–5.0] vs. 2.0 [1.0–2.0], P=0.001); the proportion of patients with functional independence was higher in those who received IVT before MT (43 [38.1%] vs. 28 [75.7%], P<0.001). However, the proportion of patients who achieved successful recanalization was similar between the two groups.
Univariate logistic regression analysis of patients with distal M1 occlusion showed that age, hypertension, diabetes, IVT before MT, baseline NIHSS score, successful recanalization, and the occurrence of HT were associated with functional independence (Table 5). In the multivariate analysis, age (adjusted OR 0.91 [95% CI, 0.87–0.96], P<0.001), IVT before MT (adjusted OR 5.30 [95% CI, 1.56–18.05], P=0.007), successful recanalization (adjusted OR 7.71 [95% CI, 1.70–34.95], P=0.008), and the occurrence of HT (adjusted OR 0.33 [95% CI, 0.13–0.87], P=0.025) were significantly associated with functional independence at 3 months. Figure 1 shows the distribution of mRS scores at 3 months according to the use of IVT before MT in patients with proximal or distal M1 occlusion.

Univariable and multivariable logistic regression analysis for functional independence in patients with distal M1 occlusion
Discussion
This study demonstrated that the use of IVT before MT was associated with improved functional outcomes in patients with emergent MCA M1 occlusion. Moreover, this study showed that the effect of IVT before MT differed according to the location of MCA occlusion. Although IVT before MT showed no significant effects in patients with proximal M1 occlusion, it was associated with functional independence in those with distal M1 occlusion. Our study showed a possible interaction between IVT before MT and the location of M1 occlusion.
Previous studies have reported that a large clot volume was a predictor of poor recanalization after IVT [9,17,18]. Because the diameter of the proximal M1 was larger, the clot occluding the proximal M1 segment might also be larger. Moreover, as clots occluding the proximal M1 were usually longer, the lytic effect of IVT could be limited [13,19-21]. Additionally, distal vessel clots in intracranial LVO were usually more permeable than those in proximal vessels; higher clot permeability could maximize the lytic effect of IVT because a larger surface area of the clot can come into contact with the tPA [8]. Our findings were in line with the results of previous reports. Among the 26 study patients with spontaneous recanalization as per cerebral MR angiography before MT, 18 (69.2%) had distal M1 occlusion while 8 (30.8%) had proximal M1 occlusion. In addition, one study showed that IVT before MT was associated with the thinning of fibrin layers and a lower fibrin composition [10,22]. Lastly, the IVT-induced MCA recanalization rate differed according to stroke subtype; early recanalization was more common and complete in patients with cardioembolic stroke than in those with other subtypes [23].
In our results, there was no significant difference in the recanalization rates according to the location of the M1 occlusion and the use of IVT before MT. Thus, the effect of IVT before MT may not only be explained by thrombolysis on recanalization. Instead, IVT before MT was associated with better functional outcomes in patients with distal M1 occlusions. As the recanalization rate was similar between patients with proximal (77.0%) and distal occlusion (81.8%), this may be explained by IVT affecting the reperfusion state after recanalization.
Clot fragmentation and distal migration were often observed after or during clot retrieval; these residues may cause a no-reflow phenomenon after recanalization [24]. In previous studies of coronary artery reperfusion, distal occlusive protection devices or the use of a rheolytic thrombectomy device did not improve the no-reflow phenomenon. In contrast, aspiration thrombectomy was effective in preventing distal embolization and improving myocardial reperfusion [25-27]. These results were in line with our findings in patients with proximal occlusion; the use of a stent retriever was associated with poor outcomes, whereas contact aspiration thrombectomy or angioplasty was not.
The use of IVT before MT was associated with good functional outcomes in patients with distal M1 occlusion, independent of recanalization. Although the recanalization rate did not significantly differ according to the use of IVT before MT, the use of IVT may have affected the residual distally embolized thrombi after recanalization (Figure 2). As mentioned previously, clots with higher permeability and those of cardiac origin may be lysed more easily in the distal area, thereby preventing the no-reflow phenomenon. A previous study reported that the rate of recanalization with distal embolization after tPA administration before MT was higher in patients with distal rather than with proximal M1 occlusion. Moreover, even though spontaneous recanalization had occurred, clots could traverse within the M1 segment [28]. Also, a previous study showed that the rate of favorable functional outcomes of stroke was higher in those who received standard-dose IVT (0.9 mg/kg) before MT as compared with those who received low-dose IVT (0.6 mg/kg) before MT despite similar successful recanalization rates [29]. This suggested that standard-dose IVT might be more effective than low-dose IVT in improving collateral flow or antegrade cerebral microperfusion in patients undergoing MT for LVO. Therefore, while the effect of IVT administration before MT was limited in patients with proximal M1 occlusion, the use of IVT before MT in patients with distal M1 occlusion can affect more distal MCA segments, including M2 or M3, thereby improving distal microcirculation and preserving cerebral microvascular perfusion downstream of the major arterial occlusion [28,30].
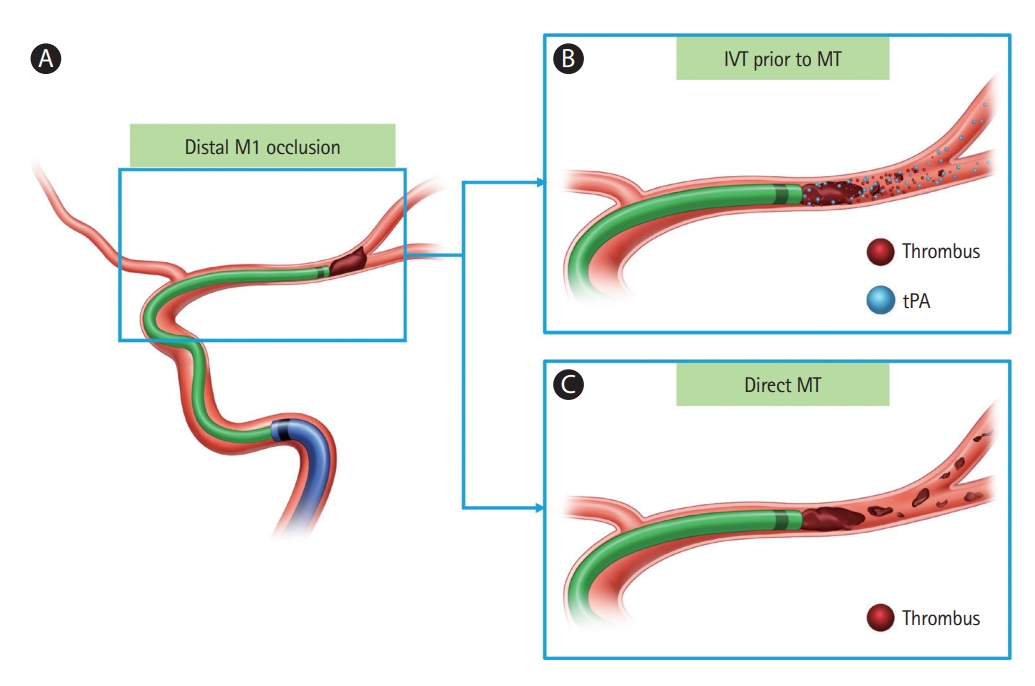
Schematic illustration of the effect of IVT before MT in distal M1 occlusion. (A) Patient with distal M1 occlusion by embolic thrombus. (B) IVT before MT can improve distal microcirculation and preserve cerebral perfusion downstream to the large-vessel occlusion. (C) Residual, distally embolized thrombi can occur frequently in direct MT. IVT, intravenous thrombolysis; MT, mechanical thrombectomy; tPA, tissue plasminogen activator.
Interestingly, although there was no significant difference in the occurrence of HT according to the use of IVT in patients with distal M1 occlusion, the proportion of HT among those with proximal M1 occlusion was higher in those who received IVT prior to MT. In proximal M1 occlusions, basal ganglia involvement was higher than in distal M1 occlusions; the lenticulostriate arteries, which are the end arteries without collateral flow, were not properly supplied by blood flow [31,32]. Moreover, basal ganglia involvement was associated with poor functional outcomes after MT [33]. Therefore, the effect of IVT before MT might be obscured by the occurrence of HT in patients with proximal M1 occlusion.
Our study had some limitations. First, although we performed multivariate logistic regression analyses to adjust for confounding factors, there were many unaccounted clinical and periprocedural variables that could have affected the functional outcomes of stroke after MT. There were no significant differences between clinical variables except for OTPT; OTPT was not associated with the primary outcome. However, there was a clear difference in OTPT between patients who received direct MT and those who received IVT before MT in treating proximal and distal M1 occlusions. Although we could not evaluate the collateral status, patients with a slow progression were more likely in the direct MT group, potentially introducing an important selection bias in this study [34]. Second, because the study period was relatively long, there may be heterogeneity in the study population in terms of the indication for IVT or MT. Third, the clots could have migrated from the proximal to the distal M1 after IVT, but with no spontaneous recanalization. Since IVT was performed before CT or MR angiography, these migrations could have affected the results of this study. Finally, this study used a retrospective design and included only a small number of patients. Therefore, these results require further verification via prospective studies involving a larger population.
Conclusions
Despite the limitations, our results showed that the effect of IVT prior to MT differed according to the location of the MCA M1 occlusion. In patients with distal M1 occlusion, IVT before MT was significantly associated with functional independence after MT, unlike those with proximal occlusion. These results may have been due to differences in clot size and composition, as well as a higher proportion of HT in patients with proximal M1 occlusion who received with IVT before MT.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.01529.
Multivariate logistic regression analysis of functional independence in the total study population
A representative case of left distal M1 occlusion. (A) The length of the measurable ipsilateral MCA with occlusion before MT (a) was 16.68 mm. (B) After successful recanalization, the total MCA (b) was 20.82 mm. This case was classified as a distal M1 occlusion (16.68/20.82×100=80.12). MCA, middle cerebral artery; MT, mechanical thrombectomy.
Flow diagram of the study patients.
Notes
Funding statement
This research was supported by grants from the Brain Convergence Research Program of the National Research Foundation funded by the Korean government (MSIT No. 2020M3E5D2A01084576) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT No. 2020R1A2C2100077).
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: JCR, BJK, JSK. Study design: JCR, BJK. Methodology: JCR, BJK, DWK, SUK, JSK. Data collection: JCR, BK, YS, DHL, JYC. Investigation: JCR, BJK, DWK, SUK. Statstical analysis: JCR, BJK. Writing—original draft: JCR, BJK. Writing—review & editing: all authors. Funding acquisition: BJK. Approval of final manuscript: all authors.