Cerebrovascular Events in Older Patients With Patent Foramen Ovale: Current Status and Future Perspectives
Article information
Abstract
Patent foramen ovale (PFO) closure, along with medical therapy, has emerged as the therapeutic gold standard in younger (<60-year-old) patients with a PFO-related stroke for preventing recurrent events. However, PFO management guidelines lack definite recommendations for older (>60 years) patients with a PFO-related cerebrovascular event, a complex group of patients who were mostly excluded from PFO closure clinical trials. Nevertheless, several studies have shown a higher prevalence of PFO among older patients with cryptogenic stroke, and its presence has been associated with an increased risk of recurrent events. Furthermore, older patients exhibit a higher prevalence of high-risk PFO anatomical features, present inherent age-related risk factors that might increase the risk of paradoxical embolism through a PFO, and have a higher incidence of ischemic events after a PFO-related event. Additionally, observational studies have shown the safety and preliminary efficacy of PFO closure in older PFO-related stroke patients. Yet, higher rates of recurrent cerebrovascular events and new-onset atrial fibrillation were observed in some studies among older patients compared to their younger counterparts. After careful case-by-case evaluation, including the assessment of hidden potential cardioembolic sources of a cryptogenic stroke other than PFO, transcatheter PFO closure might be a safe and effective therapeutic option for preventing recurrent thromboembolic events in patients >60 years with a high-risk PFO-associated stroke. Ongoing trials will provide important insights into the role of PFO closure in the elderly population.
Introduction
The presence of a patent foramen ovale (PFO) has been identified as an important source of cardioembolic cerebrovascular events in younger (<60 years old) patients and multiple randomized trials have shown the efficacy of transcatheter PFO closure (vs. medical treatment alone) for preventing recurrent ischemic stroke events in such patients [1-5]. Still, current national and international clinical PFO management guidelines lack definite recommendations about transcatheter PFO closure for patients >60 years [6-11]. However, observational data have shown a higher prevalence of PFO among older patients diagnosed with cryptogenic stroke [12,13], and the presence of PFO in the elderly population has been associated with an increased risk of recurrent cerebrovascular events [12,14-16]. Also, several studies have shown the safety and preliminary efficacy of transcatheter PFO closure in older cryptogenic stroke patients [17-21]; nevertheless, in some of these studies, a higher rate of recurrent thromboembolic events and new-onset atrial fibrillation (AF) episodes at followup have been observed following PFO closure in older patients compared to their younger counterparts, highlighting the differences and increased complexity of cerebrovascular events in this population [17,21]. This review provides an overview of (1) the epidemiology of ischemic stroke in the elderly, particularly cardioembolic and PFO-related stroke, (2) the limitations of PFO causality risk scores and ischemic stroke classification systems in the elderly, and current clinical guidelines recommendations about PFO closure in older patients, and (3) current clinical evidence regarding transcatheter PFO closure in older patients, ongoing clinical trials and future perspectives in the field.
Ischemic Stroke Epidemiology in the Elderly Population
In the United States, each year, approximately 795,000 people experience a new or recurrent stroke. Approximately 610,000 of these are first attacks, 185,000 are recurrent attacks, and most importantly, 87% are ischemic strokes [22]. Ischemic stroke incidence increases with age, especially in women ≥50 years [23]. The lifetime risk for an incident stroke is 14.5% for a person 65 years of age [22], and its mortality ranges from 74.6 to 254.2 per 100,000 in a 65- to 84-year-old population [22]. Overall, one-third of ischemic strokes have a cardioembolic source [8], and this proportion increases with age [24].
Classification of Ischemic Stroke
According to the latest stroke management guidelines, in the overall population, the distribution of ischemic stroke causes is 23% lacunar (small vessel disease) and 77% non-lacunar. Among the non-lacunar strokes, 45% are cryptogenic and 35% cardioembolic [8], with the latter (including PFO-related strokes) showing an increasing temporal trend among white people (2.4% annually between 1993 and 2015, 95% confidence interval [CI], 0.6%–4.3%) [22]. Interestingly, among the large proportion of patients with cryptogenic stroke, 50% are considered as an embolic stroke of undetermined source (ESUS), implying that the stroke is embolic in origin but with an unknown source of the thrombi, and the other 50% are considered non-embolic or non-ESUS [8]. Besides a PFO, other potential cardioembolic sources include valvular heart disease, atrial fibrillation, left ventricular disease, malignancy, aortic arch atherosclerotic plaques, as well as other intracardiac shunts [24]. Despite this, it is estimated that PFO is likely responsible for approximately 5% of all ischemic strokes and 10% of those occurring in young and middle-aged adults [25]. However, the position of PFO inside stroke classification systems can be very overlapping or confusing, leading to not-so-accurate statistics in PFO-related stroke patients. In an effort to overcome such limitations, based on all stroke classification systems limitations and the latest evidence around PFO, Elgendy et al. [25] recently proposed the term PFO-associated stroke to “all patients presenting with superficial, large deep, or retinal infarcts in the presence of a medium-risk to high-risk PFO and no other identified likely cause.” [25].
PFO and Its Role in Ischemic Stroke in the Elderly
Overall and age-specific prevalence of PFO among healthy subjects
The spontaneous closure of foramen ovale usually occurs within the first year of life but only in three-fourths of the population, leaving approximately 25% [26,27] of the adult population with a PFO, which translates to about 1.9 billion people globally [28]. The prevalence of PFO differs according to patients’ age, from 34.3% during the first three decades of life to 20.2% during the 9th and 10th decades [26]. Additionally, in autopsy studies of healthy hearts, the PFO size is larger in the 10th decade of life compared to the 1st decade of life [26], which is clinically relevant since large-size PFOs have a stronger causal relationship with a PFO-related stroke [3].
Prevalence of PFO among younger and older patients with cryptogenic stroke
In young patients (<55 years) diagnosed with cryptogenic stroke, multiple studies have shown an increased prevalence of PFO compared to their stroke of known origin counterparts [29,30], as much as four times higher than patients with stroke of known causes [30] among all diagnostic modalities [27].
Although the initial effort in the search for an association between a PFO and cryptogenic stroke among the elderly population failed (vaguely defined as having a stroke without a predetermined cause) [31], more recent studies have shown an increasing association within this population. Alsheikh-Ali et al. [29] performed a systematic review identifying 23 case-control studies, showing an OR of 2 (>1 to 3.7) for PFO in patients older than 55 years diagnosed of cryptogenic stroke. However, one important limitation of that study was the diagnostic method of PFO since in 74% of the studies was transesophageal echocardiogram (TEE), but in 26% was transcranial Doppler. More recently, Handke et al. [12] reported a TEE prevalence of PFO in 28.3% of older patients with cryptogenic stroke, compared to 11.9% in patients of the same age with a known cause of stroke. In the multivariable analysis, PFO was independently associated with cryptogenic stroke in the younger and older groups. Likewise, in a population-based study nested in the Oxford Vascular (OXVASC) study, compared to patients with transient ischemic attack (TIA) or stroke of known cause, patients with cryptogenic events had a higher prevalence of right-to-left shunt in the overall population (odds ratio [OR] 1.93, 95% CI: 1.32–2.82; P=0.001), but remarkably, this was even higher among patients >60 years (OR 2.06, 95% CI: 1.32–3.23; P=0.001) [13]. One important limitation was that the PFO prevalence was evaluated by contrast-enhanced transcranial Doppler. The authors extrapolated their results amongst the British population, estimating that every year 8,477 cryptogenic cerebral ischemic events could be attributed to significant right-to-left shunting, with 70% of them occurring in patients older than 60 years.
Pathophysiology of PFO in embolic stroke
Two main potential mechanisms can explain the role of PFO in a systemic arterial embolism. Firstly, paradoxical embolism, meaning the passage of a right-sided venous thrombus to the systemic circulation through the PFO, especially during the transient or persistent elevation of right-heart pressures [32]. This mechanism will only need a PFO gap as small as 1–3 mm to cause either severe impairment or a large infarct since 1 mm is the average size of any major cerebral cortical branch and 3 mm is the size of the middle cerebral artery stem [33]. The second mechanism would be in situ PFO thrombus formation, which can lead to an embolization into the arterial circulation [34].
High-risk anatomical PFO features
Although a PFO can only be a bystander in the settings of an otherwise cryptogenic stroke, several morphologic characteristics have been described as being more prone to a higher risk for paradoxical embolization or in situ thrombus formation, thus, with a higher likelihood of causal relationship. These morphologic characteristics include a large PFO (maximum separation of the septum primum from the secundum >2–3 mm) [35-37], long tunnel (>10 mm) [38], atrial septal aneurysm (ASA) (usually defined as hypermobility of the septum with >10 mm excursion) [14,31,36,39], shunt size [14,37,39,40], and prominent Eustachian valve [41]. Identifying these high-risk PFO features is crucial since there is potentially a higher benefit from PFO closure among patients with high-risk anatomical PFO features [42].
Moreover, score systems based on these high-risk anatomical characteristics have been developed to help proper patient selection for closure therapies [38,43]. Identifying these high-risk PFO features is important, as a recent meta-analysis from randomized trials showed that there is potentially a higher benefit from PFO closure among patients with high-risk anatomical PFO features [44], which according to observational studies on PFO closure in younger versus older patients, these high-risk features are more prevalent among the elderly population undergoing PFO closure due to a PFO-related cerebrovascular event [17,18,21].
Assessing PFO causality role: limitations of risk scores in the elderly
The risk of paradoxical embolism (RoPE) score allows an evaluation of the potential causal role of a PFO in any given patient with a stroke of unknown cause [45]. It mainly uses the patient’s age, with younger patients having an increased risk of causality, the absence of atherosclerotic risk factors, and infarct topography, to determine the likelihood that a PFO is causally related to stroke in individual patients [45]. This score has been validated and is used in clinical practice and PFO research [46].
According to the RoPE score, the higher the score, particularly above 6 points, the stronger the link of causality between a PFO and a cryptogenic stroke. Also, as per RoPE score, younger patients and patients without traditional cardiovascular risk factors have the highest PFO-attributable fraction [45]. For example, a 29-year-old non-smoker patient with a PFO and a cryptogenic cortical stroke, without hypertension, diabetes, and no history of stroke/TIA, has a RoPE score of 10 points (maximum score) and a PFO-attributable fraction of 88%. However, any patient ≥50 years with a history of hypertension, diabetes, stroke, and smoking, even in the presence of an embolic-like stroke on imaging, has a RoPE score of 3 points, meaning a PFO-attributable fraction of 0%, basically discarding any possible association between a stroke and a PFO [45], an estimation that can be misleading in the elderly population. Moreover, in this scoring system, for every 10-year increase in patients’ age, there is a decrease in the total RoPE score, and in patients with ≥70 years, the age factor adds 0 points; thus, the possible association between a PFO and an index ischemic stroke is even less likely just by the age. Additionally, based on the RoPE original dataset, Thaler et al. [14] showed that older age was a significant predictor of recurrent stroke among patients with a low (<6) RoPE score (hazard ratio [HR] 1.47, 95% CI: 1.18–1.83).
Trying to overcome some RoPE score limitations, the PFO-Associated Stroke Causal Likelihood (PASCAL) Classification System emerged to further assess the potential causal role [25] by combining the RoPE score with the most consistent high-risk PFO anatomical features to provide a more precise causal correlation. Compared to the conventional RoPE score mainly focused on patient age and other vascular risk factors, PASCAL classification with the addition of the anatomical features of PFO (namely ASA and shunt severity) showed a better performance in distinguishing subgroups who may benefit from PFO closure from those unlikely to benefit from it; thus, selection of patients with ESUS and high-risk PFO features seems to be the first step to expect a significant reduction of recurrent events. However, although the PASCAL classification system is already validated [47], it is still limited among older patients since it is based on the RoPE score, which includes age as one of the main variables.
Recurrent cerebrovascular events after an index PFO-related stroke in the elderly
According to meta-analyses, patients with a PFO-related stroke, the annual recurrence rate on medical therapy ranges from 0% to 5.8% for stroke and from 0% to 14% for stroke or TIA [48,49]. Several factors have been associated with a higher risk of recurrent events, such as older age, presence of ASA, use of acetylsalicylic acid (compared to oral anticoagulation), thrombophilia, stroke as index event, and PFO size [7].
Remarkably, compared to younger patients, elderly patients are at higher risk of recurrent cryptogenic stroke after an index PFO-related event. According to the latest European guidelines on the management of patients with PFO, based on four studies with a total of 2,171 patients [12,14-16], older patients are at an increased risk of recurrent cerebrovascular events after an index PFO-related cryptogenic stroke; among other factors, older age has an HR of 1.47 (95% CI 1.2–1.8) for cryptogenic stroke recurrence in the presence of PFO [7]. In an analysis of the CLOSE (Patent Foramen Ovale Closure or Anticoagulants Versus Antiplatelet Therapy to Prevent Stroke Recurrence) and DEFENSE-PFO (Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High-Risk Patent Foramen Ovale) trials, one of the variables associated with time to recurrent stroke was age, with a crude HR of 1.52 (95% CI 1.17–1.97, P=0.001) per 10-year increase [39]. Interestingly, another study in patients with ESUS showed that older patients had twice recurrent embolic strokes after a median follow-up of 3 years, and, when analyzed by potential embolic sources, this difference was mainly driven by PFO-related strokes among the older population (recurrent embolic stroke 12.5% vs. 5.6% in young patients with PFO-related stroke) [50].
The most recent evidence on recurrent events was reported by Mazzucco et al. [51]. The authors showed that the absolute risk of recurrent ischemic stroke in the overall population was 2.0 per 100 patient-years (95% CI, 1.57–2.55), based on 268 recurrent events during 15,058 patient-years. After analyzing by age groups, the pooled ischemic stroke risk in patients of 60 years or older was 3.27 per 100 patient-years (95% CI, 2.59–4.13). Remarkably, the authors performed another pooled analysis of four studies reporting ischemic stroke risk stratified by age in patients with cryptogenic TIA/stroke with PFO versus patients without PFO, finding that the association between PFO and stroke risk also increased with age (<65 years: OR, 0.8; 95% CI, 0.4–1.5; ≥65 years: OR, 2.5; 95% CI, 1.4–4.2; P=0.001).
The cause behind the recurrent strokes in older patients with PFO left under medical treatment remain unclear since the data on this subject is very limited. In the DEFENSE PFO trial, all recurrent strokes were considered as PFO-related [52]. Additionally, observational data shows recurrent strokes being cryptogenic even with negative AF after prolonged cardiac monitoring [20]. In observational PFO closure studies, recurrent stroke rates are lower and importantly, the main causes are atherothrombotic, AF-related, vertebral dissection and small vessel disease, among others, with the most strongly associated factors being diabetes and chronic renal disease [18,21]. Ongoing trials will provide further details on the cause of recurrent stroke in these patients.
Special considerations in elderly patients with a PFO-related stroke
The causal relationship between a PFO and a stroke among elderly patients was once thought to be lower as the incidence of stroke due to other causes increased. Nevertheless, this causal association may be higher than expected. Autopsy studies including healthy hearts showed that PFO size is larger in the last decades of life [26] with an increase in mean PFO size from 3.4 mm in the first decade to 5.8 mm in the 10th decade of life [26], and the larger the PFO, the higher the risk of having a PFO-related stroke [38].
Another special consideration is the increased risk of venous thromboembolism. The risk of deep venous thrombosis (DVT) is strongly associated with age [53], and its overall incidence has been increasing over time, particularly among elderly patients [54], also including those cases with silent thrombus formation [55]. When a DVT develops, it can serve as the nidus for a barrage of thromboemboli directed at the right atria, with 300 to 50,000 emboli per hour [56]. Both DVT and pulmonary embolism are essential considerations since, in these patients, the presence of PFO is associated with ischemic brain lesions, both at baseline and at follow-up [57].
It is known that right ventricular systolic and diastolic function, as evaluated by transthoracic echocardiogram, decreases gradually among the healthy elderly population [58], data that has been recently refined by cardiac magnetic resonance imaging [59]. Although not directly proven, having increased filling pressures in the right ventricle could increase the risk of a right-sided thrombus migrating to the systemic circulation through an interatrial shunt, especially during transitory pressure elevation in the right side of the heart observed in the Valsalva maneuver.
PFO Closure in Young Patients and Current Clinical Guidelines Recommendations
Transcatheter PFO closure has been established as an ambulatory (same-day discharge), straightforward interventional procedure [7]. The intervention has a very high safety profile, with <1% of major periprocedural complications and a 2%–6% rate of AF episodes (usually transient) within the days to weeks following the procedure [60].
In patients with cryptogenic stroke, up to four randomized trials have shown a significant reduction in recurrent ischemic stroke events following transcatheter PFO closure [1-5]. All randomized trials in the PFO-stroke field excluded patients older than 60 years, except one [3], meaning that most patients randomized in these trials were younger than 60 years. Therefore, compelling scientific evidence exists for transcatheter PFO closure in managing younger patients with cryptogenic stroke. Table 1 summarizes the randomized clinical trials on PFO closure versus medical therapy, including their age inclusion criteria and the mean age for each treatment group.

Randomized clinical trials on patent foramen ovale closure versus medical treatment in patients with cryptogenic stroke
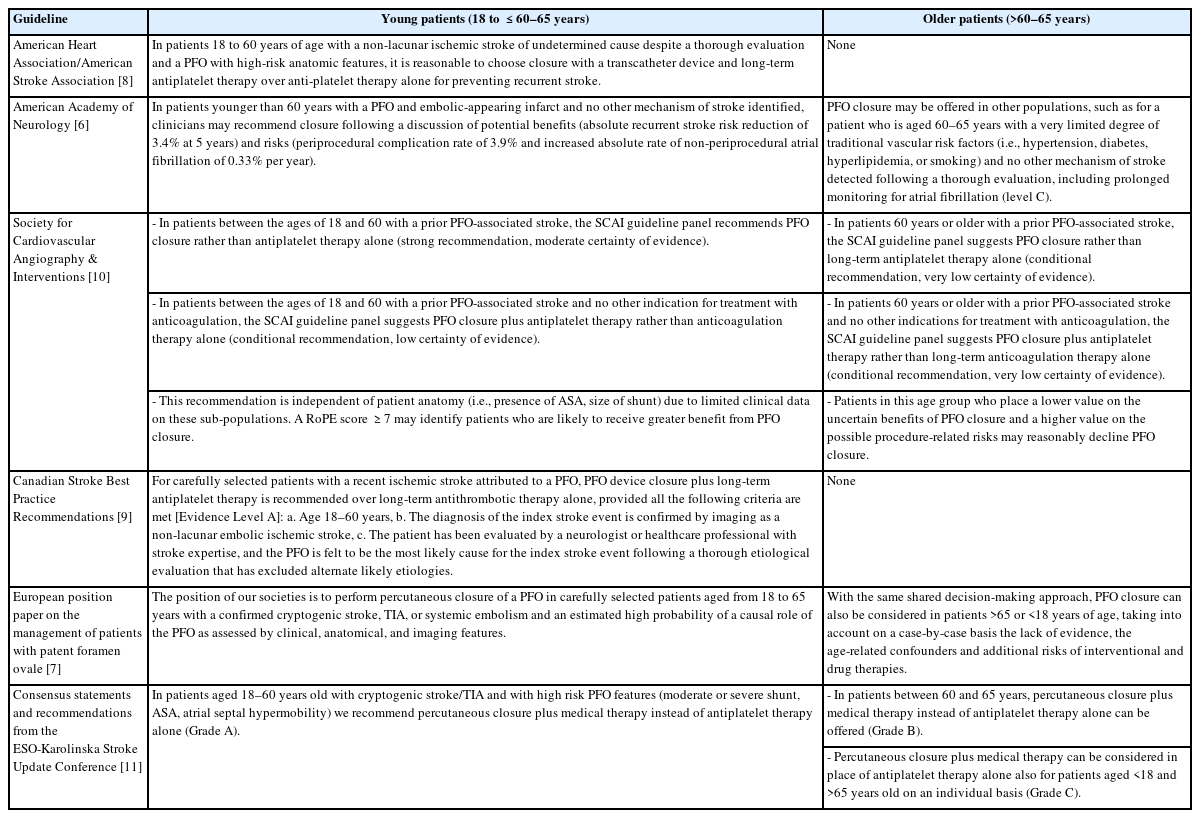
Every major clinical guideline on the management of patients with a PFO-related event recommends performing transcatheter PFO closure over medical therapy alone for patients aged 18 and ≤60–65 years with a PFO-related stroke. However, some discrepancies are observed regarding the recommendations in patients older than 60–65 years. While some guidelines are stringent and do not recommend (class III) the closure for patients >60–65 years old, others emit limited or reserved recommendations, with the possibility of a case-by-case clinical decision strategy in such cases. Table 2 summarizes the most recent and important clinical guidelines on PFO-related stroke management, emphasizing age-related recommendations and transcatheter PFO closure in younger versus older patients [6-11].
Evidence of PFO Closure in Older Patients
Clinical trial sub-analysis
The DEFENSE PFO trial is the only randomized clinical trial in the PFO closure field which included a small proportion of patients older than 60 years. This trial included 120 patients between 18 to 80 years (mean age of 50 years) [3]. In a recent subanalysis comparing younger (<60 years) versus older (≥60 years) patients, the 2-year event rate difference between medical treatment versus PFO closure was 24.6% (HR, 7.36; 95% CI, 0.28–195.81; log-rank P=0.07) in patients >60 years after a median follow-up of 3 years, and it was even higher (80%) in patients ≥70 years (HR, 11.64; 95% CI, 0.43–318.81; log-rank P=0.03) [52].
Observational studies
Besides the aforementioned sub-analysis, all the evidence available on PFO closure among older patients with PFO-related thromboembolic events comes from observational studies.
Not surprisingly, all studies showed that older patients had a higher prevalence of traditional age-related cardiovascular risk factors [17,18,20,21]. Most importantly, most studies showed a higher prevalence of high-risk anatomical PFO features among older patients [17,18,21] compared to younger patients. Transcatheter PFO closure was safe in all studies, with a very low complication rate between groups, except for one study that reported a higher incidence of vascular complications in the elderly population [17].
Overall, the mean incidence of recurrent stroke events following PFO closure in older patients (excluding ancient studies with discontinued PFO closure devices) has been about 1.38 per 100 patients-years (from 0.55 to 2.67 per 100 patients-years) [17-21], which appears to be lower than 3.27 per 100 patient-years, which is the reported incidence of recurrent cerebrovascular events in studies of older patients with PFO treated medically [51].
One important confounder in studies involving older patients with ischemic cerebrovascular events is the role of AF (frequently silent episodes) as the primary source of thromboembolism and the risk of PFO closure procedure-related AF. As a consequence, the workup of any cryptogenic ischemic cerebrovascular event includes prolonged cardiac monitoring for the detection of episodes of symptomatic or silent AF [8]; however, the optimal duration of post-stroke cardiac monitoring remains unknown [61]. A recent meta-analysis [61] including 8 studies (5 randomized clinical trials) showed that prolonged post-stroke cardiac rhythm monitoring after an ischemic stroke or TIA could lead to higher rates of AF detection and anticoagulant initiation. Nevertheless, the authors concluded that there was no solid evidence from randomized trials supporting prolonged cardiac rhythm monitoring for the prevention of recurrent stroke events.
The next clinically challenging question is how often occult AF is causally related to cryptogenic ischemic stroke. Chaisinanunkul et al. [62] identified, in a systematic review, all case-control and cohort studies on patients with identical long-term monitoring techniques in patients with cryptogenic and know-source strokes. After performing a random-effects meta-analysis applying Bayes’ theorem to determine the probability of silent AF being causal or incidental, they concluded that in patients with cryptogenic stroke, when occult AF is found, it is causal in about 38.2% of patients. Remarkably, after age stratification, occult AF detected in patients with cryptogenic stroke was considered causal in 62.3% (95 CI, 0%–87.1%) of patients under the age of 65 years and only in 28.5% (95 CI, 0%–63.7%) of patients aged 65 years and older.
In a recent meta-analysis in the younger population undergoing PFO closure, the incidence of AF after PFO closure was 3.7 per 100 patient-years (95% CI: 2.6–4.9), which was higher than the incidence of 0.1 per 100 patient-years (95% CI: 0.0–0.4) observed in patients treated medically (HR: 5.3, 95% CI: 2.5–11.41), P<0.001) [60]. Interestingly, the increased risk of AF development occurred mainly in the first 45 days post-procedure, and according to a meta-regression analysis, a higher risk was observed in older patients (P=0.001).
Observational studies on PFO closure in the elderly population have reported a rate of de novo symptomatic AF ranging from 2% to 9.2% versus 0.7% to 8.4% among their younger counterparts [17-21]. While seven studies reported no impact of age in the incidence of AF after PFO closure, two studies showed a higher rate of post-procedural AF among older patients [21,63]. Overall, before PFO closure, only one study excluded patients with prior evidence of AF [17], while the others did not exclude or mention the rate of patients with a history of AF before the procedure. Several limitations on detection methods of AF before and after the procedure and the unknown rates of asymptomatic AF would preclude drawing definite conclusions from these studies in terms of AF before and after PFO closure in older patients with a PFO-related stroke.
A potential approach for PFO closure candidates older than 55–65 years with no history of AF could be an implantable loop recorder 6 months before the procedure and systematic midterm monitoring for arrhythmia detection and its corresponding treatment. Ongoing clinical trials addressing the recurrence of AF among older patients undergoing PFO closure will provide definitive insights on this important confounder among the elderly population with PFO and a cryptogenic stroke (Figure 1).
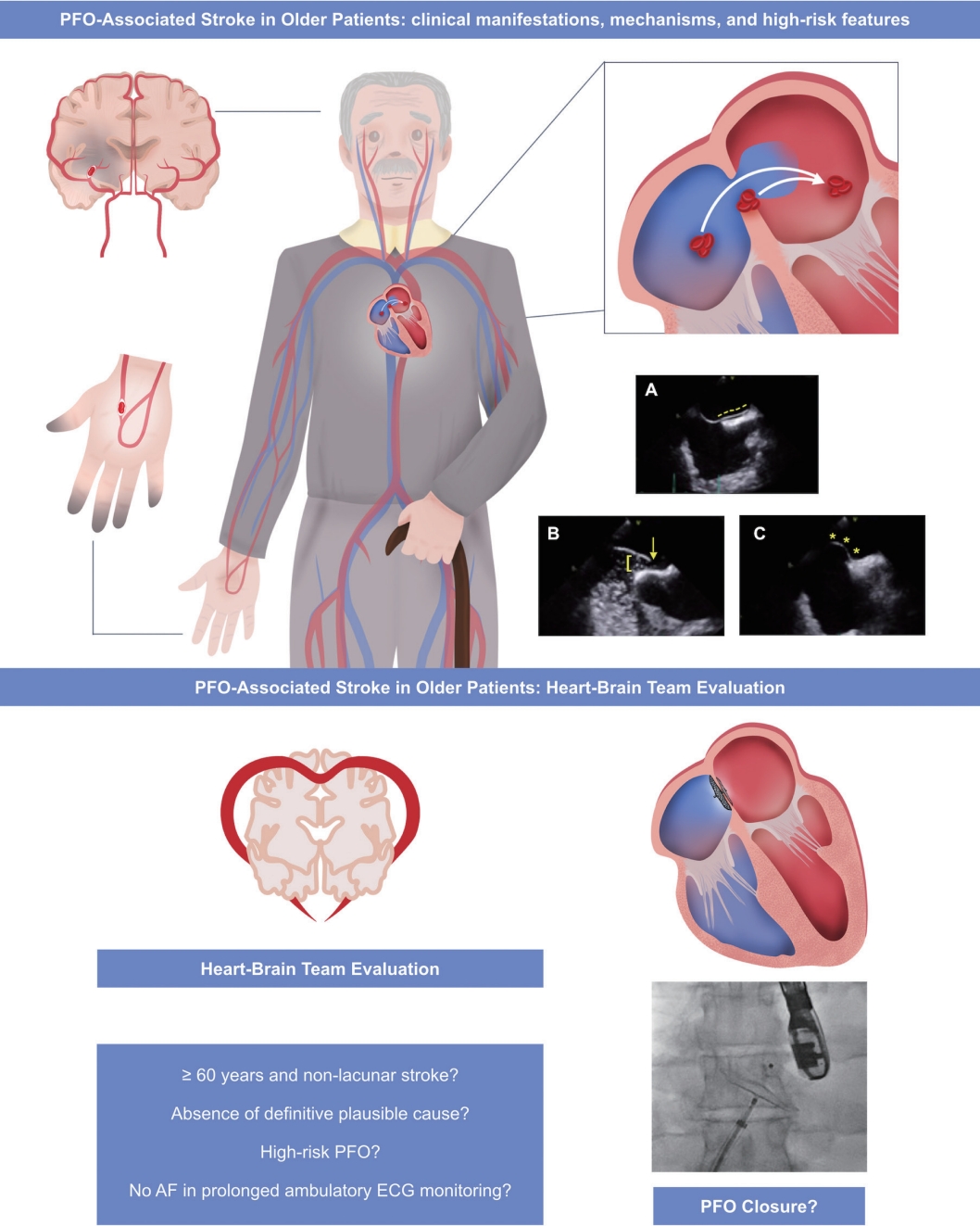
Patent foramen ovale (PFO)-associated stroke in the elderly population. The main clinical manifestations are stroke, transient ischemic attack (TIA), or peripheral embolism. This can be caused by two mechanisms: paradoxical embolism or in situ thrombus formation. The main high-risk anatomical PFO features are (A) long tunnel (dashed yellow line), (B) large PFO, >2–3 mm (yellow square bracket; yellow arrow depicting a large free passage of bubbles from the right atrium to the left atrium), and (C) atrial septal aneurysm (yellow asterisks). A heart-brain team should carefully evaluate case-by-case to assess the potential causal association between a non-lacunar stroke without any definitive plausible cause in the presence of a high-risk PFO to consider transcatheter PFO closure, ideally after discarding occult atrial fibrillation (AF) with a reasonable approach, perhaps requiring a long-term monitoring system.
Ongoing Trials on PFO Closure in Older Patients
Although some data suggest that transcatheter PFO closure is effective in older patients with a PFO-associated stroke, there are still several gaps in the evidence, namely the absence of specific randomized clinical trials in such patients. Nevertheless, there are several important ongoing studies in this field (Table 3). The CLOSE-2 trial (PFO Closure, Oral Anticoagulants or Antiplatelet Therapy After PFO-associated Stroke in Patients Aged 60 to 80 Years; NCT05387954) [64] will assess whether PFO closure plus antiplatelet therapy is superior to antiplatelet therapy alone and whether oral anticoagulant therapy is superior to antiplatelet therapy to prevent stroke recurrence in patients aged 60 to 80 years. The COACH ESUS (Prospective Registry of Elderly ESUS With PFO; NCT05238610) [65] study is a multicenter registry of elderly cryptogenic stroke patients undergoing PFO closure. The DefenseElderly study (Evaluation of Prevalence and Clinical Impact of Atrial Fibrillation in Elderly Patients With Cryptogenic Stroke and High-Risk Patent Foramen Ovale; NCT04285918) [66] will determine the incidence and clinical impact of AF and evaluate the clinical impact of AF in elderly ESUS patients (≥60 years) with no other known sources of stroke besides a high-risk PFO. Finally, one of the International PFO Consortium (NCT00859885) [67] objectives is to assess the etiological role of PFO for stroke/TIA.
Conclusions
Similar to younger patients, older (>55–65 years) patients with cryptogenic stroke present a higher prevalence of PFO than their stroke of known cause counterparts. Additionally, older patients exhibit a higher prevalence of high-risk anatomical PFO features, present inherent age-related risk factors that might increase the risk of paradoxical embolism, and have higher rates of recurrent ischemic events after an index PFO-related thromboembolic event compared to their younger counterparts. Several observational studies suggest that transcatheter PFO closure is as safe and effective for preventing recurrent ischemic thromboembolic events in older patients as in younger patients. However, ischemic stroke classification and PFO-causality score systems have significant restraints, particularly in the elderly population, and age-related recommendations by the clinical guidelines on the management of PFO are limited. Therefore, a thorough multidisciplinary case-by-case evaluation is warranted to define the best treatment option for older patients with high-risk PFO-related stroke. Treatment options for these patients could safely include transcatheter PFO closure. Nevertheless, known stroke causes, including other cardioembolic ischemic sources, should always be taken into account since they are highly prevalent in this group. Ongoing trials will provide important insights into the role of transcatheter PFO closure in the elderly population.
Notes
Funding statement
None
Conflicts of interest
Dr. Rodés-Cabau has received institutional research grants from Abbott Vascular Canada. The other authors have nothing to declare.
Author contribution
Conceptualization: JRC, JIFP. Investigation: all authors. Writing—original draft: JIFP, JRC, MR. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Acknowledgements
Dr. Rodés-Cabau holds the Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions (Laval University, Quebec City, Canada).