Chronic Lung Parenchymal Disease May Be Causally Associated With Cryptogenic Stroke With Massive Right-to-Left Shunt
Article information
Dear Sir:
Paradoxical embolism through a right-to-left shunt (RLS) has been considered a possible cause of cryptogenic stroke. Intracardiac shunts, including patent foramen ovale (PFO), are the main source of RLS, and many studies have investigated the characteristics of PFO-related strokes [1]. Meanwhile, embolic strokes secondary to an extracardiac RLS are rare, and relatively few studies have described its association with stroke. The only focused source of extracardiac RLS is a pulmonary arteriovenous malformation (PAVM) [2], and the interpretation of patients with cryptogenic stroke with massive RLS in whom neither an intracardiac shunt nor PAVM can be identified is challenging. Herein, we aimed to explore the potential extracardiac factors contributing to cryptogenic strokes with massive RLS.
This retrospective observational study was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. 2109-036-1252), and the requirement of informed consent was waived. In a subset of patients with cryptogenic stroke enrolled in our stroke registry from January 2010 to November 2021, a transcranial Doppler (TCD) ultrasound bubble study was performed. Among them, we only included patients with massive RLS to minimize potential false positives in the TCD bubble study, wherein “massive RLS” was defined by the presence of “shower” or “curtain” appearance of microembolic signals [3]. Transesophageal echocardiography (TEE) was followed by the TCD bubble study in all included patients. Patients with coexisting potential stroke mechanisms, such as small vessel occlusion, large artery atherosclerosis, cardioembolism, and dissection, were excluded.
The clinical and radiological findings of the study population were obtained. The risk of paradoxical embolism (RoPE) score and Spencer grade at rest by TCD were measured. The patterns of magnetic resonance (MR) imaging, including diffusion-weighted imaging and MR angiography, were categorized according to the criteria of previous studies [4] with modifications. Among patients with massive RLS, additional diagnostic workups, including TEE and/or chest computed tomography (CT), were performed to identify the stroke etiology. Moreover, by reviewing contrast-enhanced chest CT and medical records, the presence of chronic lung parenchymal disease was investigated, including bronchiectasis, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, and lung cancer (Supplementary Methods).
In total, 49 patients with cryptogenic stroke having a massive RLS were analyzed to determine whether the RLS had a clear source (Figure 1). The detection rate of the RLS source using TEE and/or chest CT among the patients with massive RLS was 38/49 (77.6%), with 29 patients with PFO, 6 with atrial septal defect, and 3 with PAVM. The RLS source(+) and RLS source(-) groups showed similar patterns of vascular risk factors, RoPE scores, and stroke phenotypes (Table 1). The RLS patterns did not significantly differ between the two groups. Furthermore, there were no significant differences in the D-dimer levels or the presence of frequent atrial premature complexes or enlarged left atrium between the groups.

Flowchart of patient selection. TCD, transcranial Doppler; TEE, transesophageal echocardiography; RLS, right-to-left shunt; CT, computed tomography.
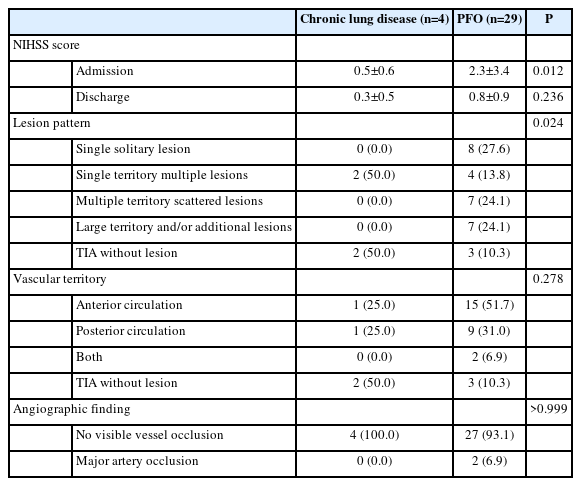
The composite of chronic lung parenchymal diseases was more prevalent in the RLS source(-) group than that in the RLS source(+) group (36.4% vs. 5.3%, P=0.018). Patients with chronic lung parenchymal diseases (bronchiectasis [n=1], COPD [n=2], and lung cancer [n=1]) in the RLS source(-) group tended to show even milder stroke severity and lesion burden than did those with PFO-related strokes (Table 2 and Supplementary Table 1).
These findings suggest that chronic lung parenchymal disease may potentially contribute to cryptogenic strokes by causing a massive RLS through vascular remodeling. In such cases, the smaller shunt size associated with this mechanism may indicate a limited passage for large thrombi, which could explain the observed milder stroke phenotypes in our cases. Previous studies have clarified the vascular remodeling process in emphysematous lungs [5] and cancer [6]. Additionally, a few epidemiological studies have shown that 21%–26% of patients with COPD have intrapulmonary RLS [7], and 55% with active cancer have RLS (intracardiac or intrapulmonary) [8]. In situations where the conventional mechanisms of cancer-related stroke [9] may not adequately account for the findings, the presence of a massive RLS resulting from chronic lung parenchymal disease may be considered as an alternative mechanism. Further research is warranted to investigate this possibility in greater depth.
The diagnostic accuracy of TCD and TEE bubble studies should also be noted [10]. Despite the diagnostic values of TEE, many studies have shown that TCD bubble studies have better sensitivity for the detection of RLS [3], probably due to the difficulties in TEE inspection. In our study, we specifically included only massive RLS cases confirmed via TCD bubble study. This was to highlight the unique subset of RLS sources demonstrating high shunt burdens but showing no PFO or PAVM on TEE and chest CT. However, the limited sensitivity of TEE in detecting extracardiac shunts, even in cases of massive RLS, might explain the low RLS source(-) detection rate. Conversely, unlike previous studies that suggested the extracardiac RLS pattern of higher bubble grade at rest compared with intracardiac RLS [10], our study did not show any significant difference in RLS patterns between the RLS source(+) and source(-) groups. This trend might be partly due to the limited number of patients and the possibility of hidden PFO.
This study had several limitations. First, as a result of including only a small number of patients who met strict criteria at a single center, the statistical power was lowered. Longitudinal studies with larger populations are warranted to elucidate how lung parenchymal diseases contribute to RLS and subsequent strokes. Second, there may be a detection bias because chest CT is not routinely performed in patients with cryptogenic stroke (chest CT was performed in 8 out of 11 patients [72.7%] in the RLS source(-) group vs. 17 out of 38 patients [44.7%] in the RLS source(+) group). Third, there may be potential issues in merging both intra- and extracardiac shunts in the comparator group. However, considering the low prevalence of PAVM, it appears less likely that it would significantly distort the overall characteristics of the entire RLS source(+) group.
In conclusion, our findings suggest that chronic lung parenchymal disease may be a potential source of RLS in patients with cryptogenic stroke.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.01074.
Representative cases showing massive RLS of undetermined source with chronic lung disease
Notes
Funding statement
This study was supported by a grant (#2022-ER-1002-00) from the Korea Disease Control and Prevention Agency.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: JSK, KHJ. Study design: JSK, KHJ. Methodology: JSK, EJL, KHJ. Data collection: all authors. Investigation: all authors. Statistical analysis: JSK. Writing—original draft: JSK. Writing—review & editing: all authors. Funding acquisition: KHJ. Approval of final manuscript: all authors.