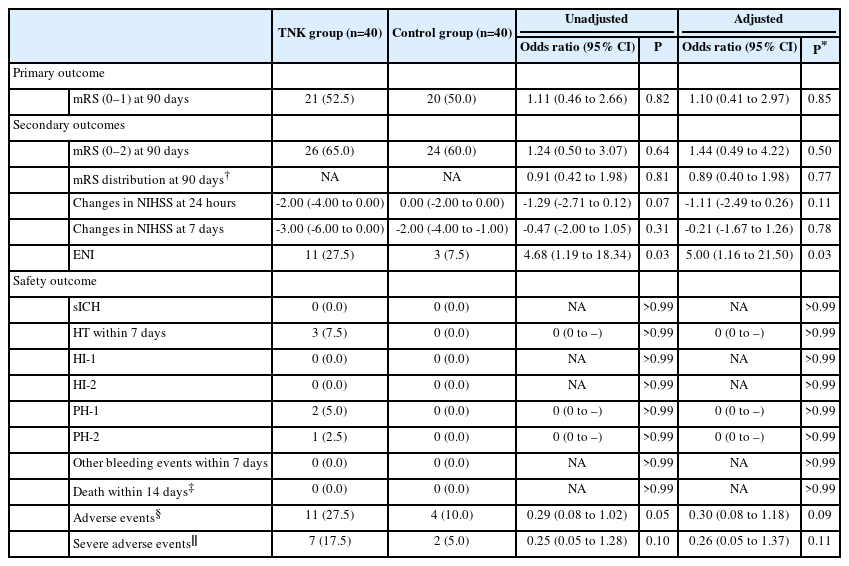
Intravenous Tenecteplase for Acute Ischemic Stroke Within 4.5–24 Hours of Onset (ROSE-TNK): A Phase 2, Randomized, Multicenter Study
Article information
Abstract
Background and Purpose
Intravenous tenecteplase (TNK) efficacy has not been well demonstrated in acute ischemic stroke (AIS) beyond 4.5 hours after onset. This study aimed to determine the effect of intravenous TNK for AIS within 4.5 to 24 hours of onset.
Methods
In this pilot trial, eligible AIS patients with diffusion-weighted imaging (DWI)-fluid attenuated inversion recovery (FLAIR) mismatch were randomly allocated to intravenous TNK (0.25 mg/kg) or standard care within 4.5–24 hours of onset. The primary endpoint was excellent functional outcome at 90 days (modified Rankin Scale [mRS] score of 0–1). The primary safety endpoint was symptomatic intracranial hemorrhage (sICH).
Results
Of the randomly assigned 80 patients, the primary endpoint occurred in 52.5% (21/40) of TNK group and 50.0% (20/40) of control group, with no significant difference (unadjusted odds ratio, 1.11; 95% confidence interval 0.46–2.66; P=0.82). More early neurological improvement occurred in TNK group than in control group (11 vs. 3, P=0.03), but no significant differences were found in other secondary endpoints, such as mRS 0–2 at 90 days, shift analysis of mRS at 90 days, and change in National Institutes of Health Stroke Scale score at 24 hours and 7 days. There were no cases of sICH in this trial; however, asymptomatic intracranial hemorrhage occurred in 3 of the 40 patients (7.5%) in the TNK group.
Conclusion
This phase 2, randomized, multicenter study suggests that intravenous TNK within 4.5–24 hours of onset may be safe and feasible in AIS patients with a DWI-FLAIR mismatch.
Introduction
Current guidelines recommend intravenous alteplase or tenecteplase (TNK) for acute ischemic stroke (AIS) within 4.5 hours of onset, but the level of recommendation for TNK is lower than that for alteplase [1,2]. Clinical trials have demonstrated the non-inferiority of TNK to alteplase in intravenous thrombolysis for AIS [3-5], as well as the higher recanalization rates and better functional prognosis of TNK than alteplase in patients undergoing bridging mechanical thrombectomy with large vessel occlusion [6,7]. Two neuroimaging-based screening randomized studies (WAKE-UP [Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke] and EXTEND [Extending the Time for Thrombolysis in Emergency Neurological Deficits]) demonstrated the benefit of intravenous alteplase for AIS beyond the time window of 4.5 hours [8,9]. Two prospective, small-sample, and non-randomized studies suggested the feasibility of intravenous TNK for AIS within 12 to 24 hours after onset [10,11]. To date, no randomized study has explored the effect of intravenous TNK in patients with AIS beyond 4.5 hours of onset.
To remedy this, we conducted this prospective, randomized, blinded endpoint assessment multicenter trial to explore the effect of intravenous TNK in patients presenting (magnetic resonance imaging [MRI]-guided) AIS within 4.5–24 hours of onset.
Methods
Study design and participants
This was a randomized, investigator-initiated, multicenter phase-2 trial with blinded endpoints, designed to evaluate the effect of intravenous TNK on AIS within 4.5–24 hours of onset. The trial was conducted at 14 hospitals (Appendix 1) in China and was authorized by the Ethics Committee of General Hospital of Northern Theater Command (approval number Y[2020]067) and all the participating sites. Signed informed consent was obtained from all participants or legally authorized persons.
Eligible patients were adults aged 18 to 80 years with acute moderate to severe ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] scores 6 to 25 at admission) who had been functioning independently in the community (modified Rankin Scale [mRS] scores 0 to 1; ranging from 0 [no symptoms] to 6 [death]) before the stroke, and were enrolled within 4.5–24 hours after onset of stroke symptoms (onset time of stroke symptoms was defined as the last well known time if it was wake-up stroke). All participants were screened using non-contrast computed tomography (CT) to exclude hemorrhagic stroke. Neuroimaging was required before randomization, as follows: (1) diffusion-weighted imaging (DWI) region: ≤1/3 of middle cerebral artery territory, 1/2 of the anterior cerebral artery territory, or 1/2 of the posterior cerebral artery territory; (2) DWI infarct volume: less than 70 mL, which was measured by an automated 3D-Slicer software (version 5.0.3, www.slicer.org); and (3) DWI-fluid attenuated inversion recovery (FLAIR) mismatch: DWI with a high signal and visually normal FLAIR. Key exclusion criteria included planned endovascular treatment, premorbid mRS ≥2, and contradiction to intravenous thrombolysis. The detailed inclusion and exclusion criteria are described in the study protocol (Appendix 2).
Randomization and masking
In this trial, eligible patients were randomly allocated (1:1) using a computer-made randomization sequence with a block size of four and sealed envelopes into either an intravenous TNK group or a control group. The final 90-day mRS score was evaluated by a qualified person who was blinded to the treatment allocation according to a standardized approach at each local site. The clinical and safety outcomes were centrally adjudicated by physicians who were unaware of the treatment assignment or detailed clinical information.
Procedures
In the TNK group, intravenous TNK (0.25 mg/kg, a maximum dose of 25 mg, CSPC Recomgen Pharmaceutical Co., LTD. [Guangzhou, China], which has the same gene and amino acid sequence as Genenthech Inc. [South San Francisco, CA, USA]) was given immediately after randomization with a single 5–10 seconds bolus. Patients in the control group received standard care, such as antiplatelets, statins, blood pressure and glucose control, and general supportive care, according to the current national guidelines for AIS [12,13].
Neurological status, measured with the NIHSS score, was assessed at baseline, 24 hours, 48 hours, 7 days, and 14 days after randomization. Detailed demographic, neuroimaging, and clinical data were obtained through randomization. Follow-ups were performed at 24 horus, 48 horus, 7 days, 14 days (or at hospital discharge, if sooner), and 90 days after randomization. Remote and on-site quality control surveillance and data validation were conducted. Central neuroimaging adjudication of the included patients was performed by a central assessor blinded to the treatment allocation (Y.J.D.).
Outcomes
The primary outcome was an excellent functional outcome (90-day mRS scores 0–1). Secondary outcomes included favorable functional outcomes (90-day mRS scores 0–2), 90-day distribution of mRS, change in NIHSS score from baseline to 24 hours and 7 days after randomization, and early neurological improvement (ENI), defined as a more than 4-point decrease in NIHSS score within 24 hours [14].
Prespecified safety outcomes included symptomatic intracranial hemorrhage (sICH) within 48 hours, defined as an increase in the NIHSS score of ≥4 points because of the intracranial hemorrhage [15], proportion of parenchymal hemorrhage (PH-1, PH-2) within 48 hours [15], proportion of hemorrhagic transformation within 7 days, any bleeding events within 7 days, and mortality within 14 days. The safety outcomes were adjudicated by the chairperson of the Data Safety Monitoring Board (X.L.S.).
Statistical analysis
Given that this was an exploratory trial, the sample size was not formally calculated and was set at 80 (40 patients per group) according to the recommendations of the Steering Committee. Statistical analyses were performed based on the intention-to-treat principle. Measurement data are represented by mean and standard deviation, if not skewed, or median and interquartile range, if skewed. Categorical data are presented as numbers (percentages). Primary analyses of the primary and secondary endpoints were not performed. The rank sum test or t-test was used for the unadjusted analysis of the measurement data, whereas the chi-square test was used for the unadjusted analysis of the categorical data. Covariate-adjusted binary logistic regression analyses were performed for all outcomes, adjusting for six prespecified prognostic factors including age, sex, systolic blood pressure, ischemic stroke, NIHSS score at presentation, and time from onset to randomization. Binary logistic regression was performed for the adjusted analysis of the primary and secondary outcomes, including favorable functional outcomes at 90 days, occurrence of ENI within 24 hours, proportion of sICH within 48 hours, proportion of parenchymal hemorrhage within 48 hours, any bleeding events within 7 days, and mortality within 14 days. An odds ratio (OR) with 95% confidence interval (CI) was calculated. The 90-day mRS score was compared using ordinal logistic regression, which was presented as an OR with 95% CI. A generalized linear model was used to compare the changes in NIHSS scores from baseline to 24 hours and 7 days after randomization, and an absolute difference with a 95% CI was calculated between the two groups. Descriptive statistics of the proportions were used as the safety endpoints. Statistical tests were considered significant when the two-sided P-value was less than 0.05. The SPSS software (version 20; IBM Corp., Armonk, NY, USA) was used for the statistical tests. This trial was registered at ClinicalTrials.gov (NCT04752631).
Data availability
Access to the data collected for the study can be obtained from the corresponding author upon reasonable request.
Results
Between March 2021 and July 2022, 166 patients were screened, and 80 participants who fulfilled the inclusion criteria were assigned to the TNK or control group (n=40, each) (Figure 1). The baseline characteristics were balanced between the two groups (Table 1), including the median age (62.68±8.87 years vs. 62.80±8.56 years), baseline NIHSS (7.50 [6.00–10.75] vs. 7.00 [6.00–8.75]), time from onset to randomization (10.97±4.67 hours vs. 11.01±4.14 hours), and baseline infarct volume (0.32 [0.00–2.28] mL vs. 0.40 [0.09–1.48] mL).

The trial flowchart. DWI, diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging.
The primary outcome occurred in 52.5% (21/40) of the TNK group and 50.0% (20/40) of the control group with no difference between groups (unadjusted OR: 1.11, 95% CI: 0.46–2.66, P=0.82; adjusted OR: 1.10, 95% CI: 0.41–2.97, P=0.85) (Table 2 and Figure 2). Regarding the secondary endpoints, an ordinal analysis of mRS distribution (unadjusted OR: 0.91, 95% CI: 0.42–1.98, P=0.81; adjusted OR: 0.89, 95% CI: 0.40–1.98, P=0.77) (Figure 2), 90-day favorable functional outcome (65% vs. 60%, unadjusted OR: 1.24, 95% CI: 0.50–3.07, P=0.64; adjusted OR: 1.44, 95% CI: 0.49–4.22, P=0.50), and changes in NIHSS score at 24 hours (unadjusted absolute difference: -1.29, 95% CI: -2.71–0.12, P=0.07; adjusted absolute difference: -1.11, 95% CI: -2.49–0.26, P=0.11) and 7 days (unadjusted absolute difference: -0.47, 95% CI: -2.00–1.05, P=0.31; adjusted absolute difference: -0.21, 95% CI: -1.67–1.26, P=0.78) did not show a significant difference, but more ENI occurred in the TNK group compared with the control group (27.5% vs. 7.5%, unadjusted OR: 4.68, 95% CI: 1.19–18.34, P=0.03; adjusted OR: 5.00, 95% CI: 1.16–21.50, P=0.03).
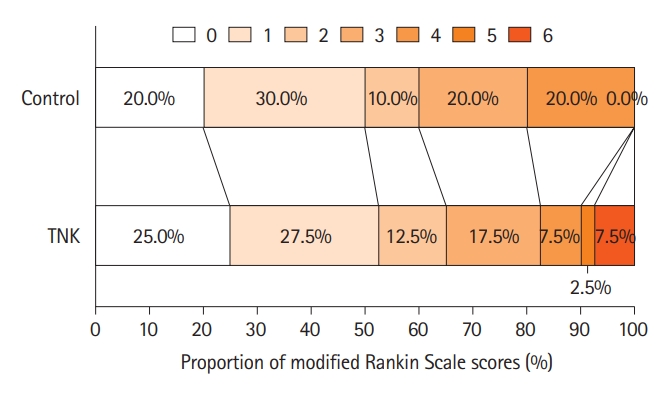
Distribution of modified Rankin Scale scores at 90 days by treatment groups in the intention-to-treat population. TNK, tenecteplase.
Intracranial hemorrhage occurred in 3 of the 40 patients (7.5%) in the TNK group and zero of the 40 patients (0.0%) in the control group, including two patients with PH-1 and one patient with PH-2 (Table 2). In this trial, there were no cases of sICH within 48 hours, other bleeding events within 7 days, or death within 14 days.
Discussion
This pilot clinical trial was conducted to investigate the effect of intravenous TNK on neurological outcomes in patients with AIS within 4.5–24 hours of onset and with a DWI-FLAIR mismatch. We found that intravenous TNK seemed feasible and may improve early neurological outcomes with a safety profile comparable to that of intravenous thrombolysis within 4.5 hours.
Intravenous alteplase is currently recommended as the standard strategy to treat eligible patients with AIS due to robust evidence [1,2,12,13]. Increasing evidence has led to the widespread off-label use of TNK to treat AIS in clinical practice [16,17]. Studies found that TNK was noninferior or superior to alteplase in intravenous thrombolysis for AIS [3,4,18,19], and produced higher recanalization rates and better functional prognosis than alteplase in patients undergoing bridging mechanical thrombectomy with large vessel occlusion [6,7]. For example, a recent study found that intravenous TNK was related to better 90-day clinical outcomes when compared with alteplase in patients with AIS [18], while two randomized trials (NORTEST [Norwegian Tenecteplase Stroke Trial], AcT [Alteplase Compared to Tenecteplase in Patients With Acute Ischemic Stroke]) did not provide any evidence supporting the superiority of TNK to alteplase. In patients undergoing bridging mechanical thrombectomy with large vessel occlusion, TASTE-A (Tenecteplase Versus Alteplase for Stroke Thrombolysis Evaluation Trial in the Ambulance) and EXTEND-IA TNK (Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke) trials found that 0.25 mg/kg TNK significantly improved recanalization and clinical outcomes when compared with alteplase [6,7]. Based on the strict neuroimaging selection, two randomized multicenter trials have provided evidence supporting the benefit of intravenous alteplase in AIS with more than 4.5 hours of symptom onset [8,9], which was further confirmed in a meta-analysis [20]. However, the effect of intravenous TNK in AIS with more than 4.5 hours has rarely been reported.
In this trial, eligible patients within 4.5–24 hours of onset were selected based on the presence of a DWI-FLAIR mismatch. The neuroimaging selection strategy is easy and has been well demonstrated to select eligible patients with stroke for reperfusion treatment [9,21,22]. The time window of 4.5–24 hours in this trial is consistent with a prospective non-randomized study [10] and several ongoing trials [23,24], but is longer than that in the WAKE-UP and EXTEND studies [8,9]. In this trial, more ENI was found in the TNK group, but 90-day functional outcome was similar in the TNK group compared with the control group with 2.5% difference, which was less than the 7% difference when considering patients extending thrombolysis to 4.5–9 hours and wake-up stroke using perfusion imaging [25]. On the one hand, this finding suggested that ENI might not be associated with the 90-day outcome. However, the failure of translation from ENI to 3-month outcome may be attributable to the small sample size. Importantly, no sICH occurred in the TNK or control group. Three patients had intracerebral hemorrhage in the TNK group, which is comparable to previous studies [4]. In addition, TNK used in the ROSE-TNK (MRI-guided thrOmbolysis for Stroke bEyond Time Window by TNK) trial was the same as that used in the TRACE-2 (Tenecteplase Reperfusion therapy in Acute ischaemic Cerebrovascular Events-2) trial [5], which has demonstrated its non-inferiority to alteplase. Collectively, these results suggest that intravenous TNK may be safe, feasible, and capable of improving early neurologic outcomes.
The strength of this trial is its randomized multicenter design to investigate the effect of intravenous TNK in MRI-guided AIS within 4.5–24 hours of onset, which has not been previously reported. The safety and possible benefits should be investigated in future trials with larger sample sizes. The main limitation of this trial was its small sample size, which might have rendered our findings inconclusive. Several ongoing trials [23,24] are attempting to determine the effect of intravenous TNK within an expanded time window 4.5 hours after stroke onset, and these results will provide more evidence. Another limitation was the open-label design of this trial; however, blinded endpoint evaluations were used to reduce bias in the assessment of the primary endpoint. Third, more than half of the enrolled population consisted of patients with wake-up stroke who were partially eligible for the WAKE-UP trial but received intravenous alteplase in the control group due to no recommendation in current Chinese stroke guidelines [12,13]. Fourth, there was a 48% (80/166) DWI-FLAIR mismatch in this trial, which was higher than that in the WAKE-UP trial (37%) [9]. The high rate might be due to the strict screening workflow protocol whereby patients suspected of hypodensity changes on brain CT did not proceed to further screening. Another possible explanation is chance randomness owing to the small sample size. Fifth, the enrolled population was not representative of the patients within an extended time window because more than half of the enrolled population had wake-up stroke. Sixth, the DWI-FLAIR mismatch selection strategy was also a limitation. It would be more reasonable to investigate this issue in non-awake patients with stroke using perfusion imaging. Finally, patients with planned endovascular treatment were excluded from this trial, which might have limited the generalizability of the current results.
Conclusion
This phase 2, randomized multicenter trial suggests that intravenous administration of TNK within 4.5–24 hours of stroke onset may be safe and feasible, with the potential to improve early neurological outcomes in patients with a DWI-FLAIR mismatch.
Notes
Funding statement
This study was funded by grants from the Science and Technology Project Plan of Liao Ning Province (2019JH2/10300027).
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: HSC. Study design: HSC. Methodology: YC. Data collection: all authors. Investigation: all authors. Statistical analysis: LW, YJD, Y.C. Writing—original draft: LW, YJD. Writing—review & editing: HSC. Funding acquisition: HSC. Approval of final manuscript: all authors.
Acknowledgements
We thank the investigators and research staff (Appendix 1) at the participating sites and the members of the trial steering and data monitoring committees (Appendix 1). We also thank the participants and their families and friends.
References
Appendices
Appendix 1.
MRI-guided thrOmbolysis for Stroke bEyond Time Window by TNK (ROSE-TNK): a prospective, randomized, blinded-endpoint, multicentre trial
jos-2023-00668-Appendix-1.pdf