Clinical and Safety Outcomes of Endovascular Therapy 6 to 24 Hours After Large Vessel Occlusion Ischemic Stroke With Tandem Lesions
Article information
Abstract
Background and Purpose
Effect of endovascular therapy (EVT) in acute large vessel occlusion (LVO) patients with tandem lesions (TLs) within 6–24 hours after last known well (LKW) remains unclear. We evaluated the clinical and safety outcomes among TL-LVO patients treated within 6–24 hours.
Methods
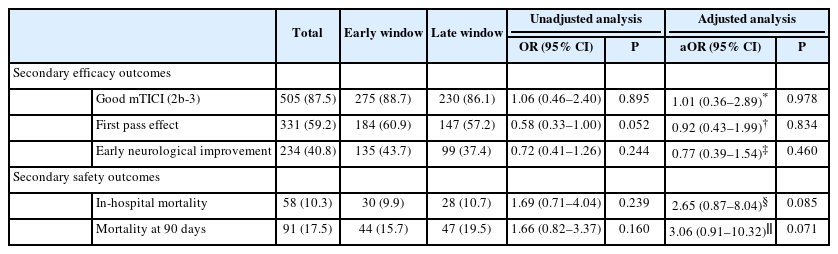
This multicenter cohort was divided into two groups, based on LKW to puncture time: early window (<6 hours), and late window (6–24 hours). Primary clinical and safety outcomes were 90-day functional independence measured by the modified Rankin Scale (mRS: 0–2) and symptomatic intracranial hemorrhage (sICH). Secondary outcomes were successful reperfusion (modified Thrombolysis in Cerebral Infarction score ≥2b), first-pass effect, early neurological improvement, ordinal mRS, and in-hospital and 90-day mortality.
Results
Of 579 patients (median age 68, 32.1% females), 268 (46.3%) were treated in the late window and 311 (53.7%) in the early window. Late window group had lower median National Institutes of Health Stroke Scale score at admission, Alberta Stroke Program Early Computed Tomography Score, rates of intravenous thrombolysis, and higher rates for perfusion imaging. After adjusting for confounders, the odds of 90-day mRS 0–2 (47.7% vs. 45.0%, adjusted odds ratio [aOR] 0.71, 95% confidence interval [CI] 0.49–1.02), favorable shift in mRS (aOR 0.88, 95% CI 0.44–1.76), and sICH (3.7% vs. 5.2%, aOR 0.56, 95% CI 0.20–1.56) were similar in both groups. There was no difference in secondary outcomes. Increased time from LKW to puncture did not predicted the probability of 90-day mRS 0–2 (aOR 0.99, 95% CI 0.96–1.01, for each hour delay) among patients presenting <24 hours.
Conclusion
EVT for acute TL-LVO treated within 6–24 hours after LKW was associated with similar rates of clinical and safety outcomes, compared to patients treated within 6 hours.
Introduction
Endovascular therapy (EVT) is considered a highly effective and safe approach for large vessel occlusion (LVO) of the anterior circulation in acute ischemic stroke (AIS), performed within 24 hours from symptom onset [1,2]. Despite the overwhelming effectiveness of EVT, there is a lack of evidence supporting its use in patients who present with AIS due to concomitant intracranial LVO and extracranial internal carotid artery (ICA) occlusions, commonly referred to as tandem lesions (TLs), within 6 to 24 hours after last known well (LKW) [3].
TLs represent up to one-third of AIS patients and are associated with poor clinical outcomes [4-8]. Multicenter retrospective registries have attempted to identify effective treatment approaches and outcome predictors for optimal patient selection treated within 6 hours after LKW [9-11]. However, literature is scarce about the impact of EVT on functional and safety outcomes among patients with TLs treated 6–24 hours after symptom onset. AURORA (Analysis Of Pooled Data From Randomized Studies Of Thrombectomy More Than 6 Hours After Last Known Well) was a patient-level meta-analysis of six clinical trials for LVO patients treated beyond 6 hours from LKW. Of these only four trials included patients with TLs [7,8,12,13]. Similarly, the Thrombectomy in Tandem Occlusions (TITAN) and Endovascular Treatment in Ischemic Stroke (ETIS) retrospective multicenter registries have also shed light on clinical questions regarding the effects of EVT among patients with acute TLs, however, both registries studied patients within 8 hours after LKW [10,11,14].
Therefore, in this study from a large multicenter collaboration, we sought to evaluate the effect of EVT on clinical and safety outcomes among TL-LVO patients treated within 6–24 hours after LKW.
Methods
The study was approved under the waiver of informed consent by the local institutional review boards at each participating center and is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [15].
Study design, settings, and participants
We used a pooled, international multicenter cohort registry for the study. Patient eligibility and data collection have been reported previously [16]. The data consisted of consecutive adult patients with anterior circulation TLs who underwent EVT within 24 hours of symptom onset, between January 2015 and December 2020, from 16 stroke centers (15 in the United States [US] and 1 in Spain). TL was defined as an intracranial LVO (petrous to terminus segments of the ICA or M1 or proximal M2 segment of the medial cerebral artery) with a concomitant extracranial ICA stenosis ≥50% or occlusion, as defined by the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria [17]. Inclusion criteria were: (1) age ≥18 years; (2) EVT for intracranial occlusion up to 24 hours; and (3) treatment for extracranial ICA lesions demonstrated on admission computed tomographic angiography (CTA), magnetic resonance angiography, and/or intraprocedural digital subtraction angiography (DSA). Perfusion imaging, including computed tomography (CT) or magnetic resonance perfusion (CTP/MRP), was based on center specific late window EVT selection protocols. Centers using perfusion imaging for selecting patients presenting in late window followed the current US and European guidelines, which recommend EVT for patients who meet the DAWN (DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo) or DEFUSE-3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3) trials’ inclusion criteria (Supplementary Table 1). Patients with isolated extracranial ICA lesions were excluded.
Study groups, data elements, exposures, and interventions
Patients were divided into two groups depending on LKW to puncture time: the early window (within 6 hours) and the late window (between 6 to 24 hours). Information on patients’ demographics, risk factors and comorbid conditions, National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), previous antithrombotic treatment, and intravenous (IV) thrombolysis administration were collected. Imaging characteristics included baseline Alberta Stroke Program Early Computed Tomography Score (ASPECTS), etiology of the extracranial ICA lesion, and site of occlusion (as determined on CTA/DSA). Stroke workflow time metrics were LKW to groin puncture, door to arterial puncture, and arterial puncture to reperfusion/end of the procedure.
Procedural variables included intracranial stenting and/or angioplasty, cervical revascularization order in reference to the ICA lesion (anterograde versus retrograde), and intraprocedural antiplatelet therapy regimens categorized as “no antiplatelets,” “single oral,” “dual oral,” and/or “intravenous antiplatelet(s)” administered immediately before, during, or immediately after the EVT procedure. The antiplatelet regimen was determined by the treating physician based on institutional protocols and included acetylsalicylic acid (ASA) (aspirin), glycoprotein IIB/IIIA inhibitors (tirofiban, eptifibatide, and abciximab), and/or P2Y12 inhibitors (clopidogrel, ticagrelor, and cangrelor). Since only one European site had the capabilities to use IV ASA (n=11), aspirin was categorized within the “single/dual oral” group for the purposes of this study. Eligible patients also received IV thrombolysis at the discretion of the treating clinician(s) in accordance with the American Heart Association (AHA) guidelines [18]. Endovascular and medical therapeutic interventions were performed according to each institution’s protocol under conscious sedation or general anesthesia and at the discretion of the interventionalists.
Outcome measures
The primary clinical outcome was the proportion of functional independence measured at 90 days using the mRS. The score was obtained by board-certified vascular neurologists during a routinely scheduled clinical visit, or by a study nurse, certified in administering the mRS, during a standardized telephone interview if the patient was unable to attend. Favorable functional outcome was defined as an mRS score of 0–2.
The primary safety outcome was symptomatic intracranial hemorrhage (sICH), as defined according to the European Collaborative Acute Stroke Study (ECASS-3) criteria [19]. Secondary safety outcomes included parenchymal hematoma type 2 (PH2) and petechial hemorrhage (hemorrhage infarction, HI1, and HI2), as per the Heidelberg Bleeding classification [20]. Other secondary outcomes were the ordinal shift of 90-day mRS, first pass effect (modified Thrombolysis in Cerebral Infarction [mTICI] 2b-3 after first pass), early neurological improvement defined as improvement in baseline to 24 hours NIHSS by >4 points, successful reperfusion (mTICI 2b-3), and in-hospital and 90-day mortality (mRS=6).
Statistical analysis
Descriptive statistics were used to summarize continuous and categorical variables. We reported categorical variables as counts and percentages and continuous variables as means (standard deviation) or medians (interquartile range [IQR]). Shapiro-Wilk test and histograms were used to assess the normality of distributions. For the univariate analysis, we used Student’s t-test or Wilcoxon rank-sum test for continuous variables and chi-square or Fisher’s exact test for categorical variables, as needed. Patients were divided into two groups according to LKW to puncture time and baseline characteristics were described.
We performed multivariable logistic regressions to evaluate the association between patients treated in the early and late window and the clinical and safety outcomes. Final models were selected using bidirectional stepwise procedures with Akaike’s information criteria and Bayesian information criteria; candidate variables were selected a priori: age, sex, race, hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, smoking status, initial NIHSS, ASPECTS, treatment with IV-thrombolysis, direct-to-angio suite strategy, mTICI 2b-3, intra-arterial tissue plasminogen activator (tPA), sICH, PH2, heparin, antiplatelet regimens, ICA treatment technique (stenting or non-stenting), ICA treatment timing (retrograde, anterograde, delayed), preprocedure ICA stenosis/occlusion, etiology of ICA lesion, and first pass effect. An adjusted ordinal regression model was also generated to estimate the odds of lower versus higher scores of mRS at 90 days. When fitting all the multivariable models, the recruiting center was included as a random effect.
Moreover, we assessed the heterogeneity of the late window effect size on favorable functional outcome at 90 days for prespecified subgroups: age, etiology, ASPECTS, periprocedural antiplatelet regimens, ICA treatment technique, and admission modality; and sICH and PH2, for the subgroups: age, ASPECTS, periprocedural antiplatelet regimens, and stenting of ICA. Finally, given the heterogeneity of imaging selection in late window patients prior to EVT, we also performed a sensitivity analysis for the primary and secondary outcomes stratified by the utilization of perfusion imaging in the triage process.
The odds ratios (ORs) and 95% confidence intervals (CIs) with P-value for the effect size of each group were computed. The resulting coefficients were considered significant at a two-sided alpha level of <0.05 set as statistical significance. All analyses and graphs were conducted using R software version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). Data will be made available upon reasonable request from the corresponding author.
Results
Of the 691 patients included in the TL registry from 16 centers, 579 met the inclusion criteria and were included in the final analysis. Of these, 311 (53.7%) were treated in the early window and 268 (46.3%) in the late window (Figure 1). The median age of the cohort was 68 (IQR 59–76) years, with a lower proportion of females (186, 32.1%). Demographics and baseline characteristics of both groups are presented in Table 1. The late window group had significantly lower median NIHSS at admission (16 [IQR 10–20] vs. 17 [IQR 13–21], P=0.013), rates of IV-tPA treatment (18.7% vs. 69.1%, P<0.001), median ASPECTS (8 [IQR 7–9] vs. 9 [IQR 7–10], P<0.001), and higher rates of perfusion imaging prior to EVT (72% vs. 43%, P<0.001), while the baseline infarct core volumes were similar (23.5±28.9 mL vs. 25.3±40.4 mL) among the two groups.
Clinical outcomes
We found no significant differences in 90-day favorable functional outcome among TLs patients presenting in the late window compared to the early window, before and after adjusting for confounders (47.7% vs. 45%, adjusted OR [aOR] 0.71, 95% CI: 0.49–1.02, P=0.065). Similarly, there were no differences in the odds of a favorable shift in 90-day mRS compared with early window patients (aOR 0.88, 95% CI: 0.44–1.76, P=0.713) (Figure 2A). Additionally, when we evaluated the association of time-to-stroke onset and favorable outcome, we found that time from LKW to puncture was not associated with the predicted probability of achieving an mRS 0–2 at 90 days (aOR 0.99 for each hour delay, 95% CI: 0.96–1.01, P=0.307), when these TLs patients were treated with EVT (Figure 2B).

Bar charts of (A) shift analysis of modified Rankin Scale (mRS) at 90 days and (B) predicted probability of mRS 0–2 at 90 days. aOR, adjusted odds ratio; OR, odds ratio; CI, confidence interval; CAD, coronary artery disease; NIHSS, National Institutes of Health Stroke Scale; IV-tPA, intravenous tissue plasminogen activator; mTICI, modified Thrombolysis in Cerebral Infarction; sICH, symptomatic intracranial hemorrhage; ICA, internal carotid artery; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; mRS, modified Rankin Scale; DTAS, direct to angiosuite strategy. *Proportional odds model: adjusted for age, sex, CAD, initial NIHSS, IV-tPA, mTICI 2b-3, sICH, ICA treatment timing, and ASPECTS; †Binomial model (mRS 0–2 vs. 3–6): adjusted for age, sex, CAD, initial NIHSS, IV-tPA, DTAS strategy, mTICI 2b-3, sICH, ICA stenting, pre-procedure ICA stenosis, first pass effect, and ASPECTS.
Safety outcomes
There were similar rates of sICH among patients treated in the late window compared to the early window (3.7% vs. 5.2%, aOR 0.56, 95% CI: 0.20–1.56, P=0.268). Similarly, there were no significant differences in the rates of hemorrhagic transformation: PH2 (6.4% vs. 8.4%, aOR 0.69, 95% CI: 0.29–1.63, P=0.394), and petechial hemorrhage (24.0% vs. 18.8%, aOR 1.53, 95% CI: 0.86–2.70, P=0.146) (Figure 3).
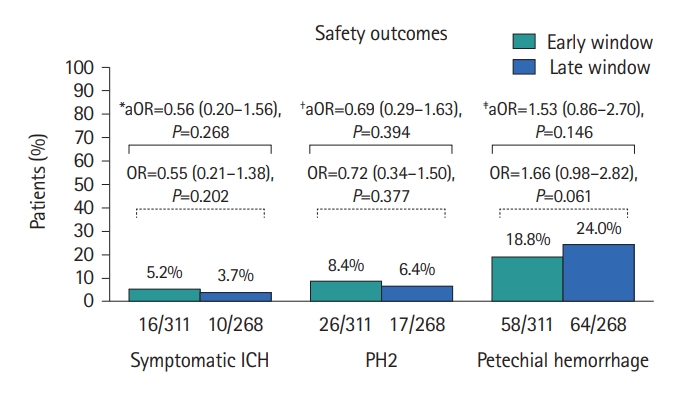
Bar chart of rates of symptomatic ICH, PH2, and petechial hemorrhage in TLs treated in the early window and late window. ICH, intracranial hemorrhage; PH2, parenchymal hematoma type 2; TLs, tandem lesions; IV, intravenous; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; ICA, internal carotid artery; OR, odds ratio; aOR, adjusted odds ratio. *Adjusted for sex, hypertension, diabetes mellitus, atrial fibrillation, direct to angiosuite strategy, IV heparin, periprocedural antiplatelets regimens, and first pass effect; †Adjusted for diabetes mellitus, atrial fibrillation, direct to angiosuite strategy, ASPECTS, IV heparin, periprocedural antiplatelets regimens, and first pass effect; ‡Adjusted for race, atrial fibrillation, ASPECTS, IV heparin, and ICA stenting.
Secondary outcomes
We did not observe any significant differences in the rates of successful reperfusion (86.1% vs. 88.7%, aOR 1.01, 95% CI: 0.36–2.89, P=0.978), first pass effect (57.2% vs. 60.9%, aOR 0.92, 95% CI: 0.43–1.99, P=0.834), and early neurological improvement (37.4% vs. 43.7%, aOR 0.77, 95% CI 0.39–1.54, P=0.460) when we compared late window TLs patients to the early window group. Similarly, the analysis of secondary safety outcomes did not show any significant differences for the rates of in-hospital mortality (10.7% vs. 9.9%, aOR 2.65, 95% CI: 0.87–8.04, P=0.085) and mortality at 90 days (19.5% vs. 15.7%, aOR 3.06, 95% CI: 0.91–10.32, P=0.071), among patients treated in late window compared to the early window group (Table 2).
Heterogeneity of treatment time effect in pre-specified subgroups
There was no heterogeneity in treatment time for 90-day favorable functional status across the pre-specified subgroups including age, ICA lesion etiology, ASPECTS, periprocedural antiplatelet regimens, ICA lesion treatment technique, and admission modality (Supplementary Figure 1). Similarly, no significant heterogeneity was observed for sICH and PH2 across the pre-specified subgroups including age, ASPECTS, periprocedural antiplatelet regimens, and ICA lesion treatment technique (Supplementary Figure 2).
Clinical outcomes of late window patients based on imaging selection
A total of 178 (72%) patients in late window group were selected using advance perfusion imaging (CTP [168, 94.3%] and MRP [10, 5.7%]), while 90 patients were selected using CT and CTA only. A sensitivity analysis comparing late window patients stratified by the use of perfusion imaging prior to EVT demonstrated no significant differences in safety and clinical outcomes including rates of PH2 (aOR: 1.68, 95% CI: 0.42–6.76, P=0.466), petechial hemorrhage (aOR: 0.98, 95% CI: 0.47–2.02, P=0.950), favorable functional outcomes at 90 days (aOR: 1.40, 95% CI: 0.74–2.64, P=0.296), successful reperfusion (aOR: 1.62, 95% CI: 0.65–4.05, P=0.305), and mortality at 90 days (aOR: 1.07, 95% CI: 0.44–2.57, P=0.885), after adjusting for the confounders (Supplementary Table 2).
Discussion
Our study observed that TLs patients treated with EVT in the late window group benefited similarly to those treated in the early window. TLs treated during the late window exhibited similar rates of achieving favorable functional outcomes at 3 months without an increased risk of hemorrhage or mortality.
The undeniable effect of time on ischemic brain tissue and the urgency of achieving early reperfusion have led to a better selection of patients who benefit the most from EVT. Initially limited to the first 6 hours from stroke onset, the late window trials have demonstrated that selected patients should be treated up to 24 hours, as shown by HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) and AURORA meta-analyses that these late window patients achieve similar functional independence at 90 days (45.9% vs. 46%) without significantly increased risk of sICH (5.3% vs. 4.4%) [1,2,12,21]. The HERMES meta-analysis, which observed a greater benefit of EVT over medical therapy, included 122 patients with TLs, thereby extending the observed beneficial effect of treatment to the TL population [1]. In contrast, the 27 TLs patients included in the DAWN trial were excluded from the acute stenting treatment [12].
Recently it has been shown that besides time, brain autoregulation is crucial for the evolution of ischemic injury, which can extend up to approximately 72 hours from the stroke onset [22]. Moreover, it varies among individuals and is affected by the hemodynamic and metabolic changes during the acute phase of ischemia, depending on the individual’s anatomical and physiological characteristics [23,24]. This has led to the differentiation of fast (patients whose infarct core increases rapidly) and slow progressors (those who show a slower infarct core evolution), respectively [25]. It is believed that slow progressors are more resilient to ischemic injury than fast progressors, thereby achieving a greater benefit of treatment in the late window [25]. However, a decrease in carotid blood flow in TLs patients secondary to atherosclerotic disease may indeterminately affect brain autoregulation. Furthermore, existing literature suggests an increased infarct growth rate in the presence of a carotid lesion due to impaired collateral circulation may exacerbate hypoperfusion leading to an increased infarct progression and consequently reducing the clinical benefit of EVT [26,27]. Our results further support this observation demonstrating that these late window TL patients had a lower median ASPECTS (8 vs. 9), and similar (23.5 mL vs. 25.3 mL) infarct core volumes in patients who underwent perfusion imaging at admission (56.7%) [24]. In fact, chronic hypoperfusion state with exhausted compensatory reserved may be present at intracranial vasculature at the time of stoke in patients who cannot develop sufficient anterograde compensation from an anatomically robust anterior or posterior communicating artery [28].
In our study, we did not find a significant difference in the underlying demographics, etiology, cervical carotid approach, and the use of antiplatelet therapies. Patients treated in the early and late window showed similar rates of ICA treatment with acute stenting (61.1% vs. 58.1%), atherosclerotic etiology of the lesion (75.8% vs. 80.9%), periprocedural antiplatelet regimens (no antiplatelets: 22.2% vs. 20.1%, single oral regimen: 19.3% vs. 23.5%, dual oral regimen: 31.5% vs. 22.8%, and intravenous antiplatelets: 27.0% vs. 33.6%), and ICA occlusion status (48.1% vs. 56.4%). Although, as expected, the early window patients had significantly increased rates of IV thrombolysis (69.1% vs. 18.7%) compared to the late window. Nevertheless, the rate of hemorrhagic complications were similar in both groups, despite a higher proportion of patients undergoing acute stenting and antiplatelet therapy and adjusting for common confounders (including the use of heparin, reperfusion status, NIHSS, and ASPECTS). Although, we did not find any differences in the outcomes among the late window patients, there was an increased proportion of patients who received an advance imaging (43.0% vs. 72.1%) in the late window group, reflecting a more careful selection of these patients by the treating interventionalists based on current late window guidelines. These results, combined with current evidence supporting the use of acute stenting in TLs contrasts with results from an international survey in which a large proportion of stroke experts reported concerns about the use of acute stenting and antiplatelet therapy in TLs patients receiving tPA [29,30]. Moreover, it triggers an important question that may merit further research in this subgroup.
The absence of significant differences in the treatment effect for both populations indicates that interventionalists should not differ in endovascular treatment for these patients. The overall rates of good functional outcome (mRS 0–2) at 90 days as well as sICH in the late window group in this study are similar to the pooled results of AURORA (mRS: 49% vs. 45.9% and sICH: 4.1% vs. 5.3%, respectively). Moreover, our late window group exhibited higher rates of functional independence than the population included in the TITAN multicenter collaboration (57% vs. 38.3%) [2,11,31]. Additionally, rates of petechial hemorrhage and PH2 are not higher to those reported in previous thrombectomy studies. Although this study provides evidence of treating AIS patients with TL regardless of time, there is a need for prospective studies to validate the effect of treatment time, as well as to elucidate the optimal treatment approach and patient selection methods [11,32].
Strengths and limitations
Our findings should be interpreted within the context of the following limitations. Although this study has the inherent limitations of observational data, a large sample size from prospectively maintained databases adds to its robustness. Moreover, due to the retrospective design and lack of treatment guidelines, there may be some heterogeneity for the patients included and excluded in each group. In fact, the use of admission perfusion imaging was nearly 72% in the late window and 43% in the early window, supporting that most patients were selected following current guidelines and therefore exhibited different collateral status and infarct growth rates. Nevertheless, as observed in the isolated LVO pivotal studies, the treatment effect of TLs remains consistent in both late and early window populations when selected following current guideline criteria. Furthermore, there may be some reporting bias due to lack of core lab adjudication and unblinded follow-up of patients. Lastly, despite multiple imputations and controlling for confounders, missing data and lack of sample size calculations may have also impacted our results estimation.
Conclusions
In this multicenter, international cross-sectional study, EVT for acute TL-LVOs treated within 6–24 hours after LKW was associated with similar rates of clinical and safety outcomes compared to patients treated within 6 hours. While there was difference in the severity of presentation, admission infarct volumes estimated by ASPECTS and uniform use of perfusion protocols, patients with AIS due to TLs who present in the late window may benefit from endovascular treatment as much as those presented in the early window. Moreover, clinical and safety outcome rates were found similar to those in patients with isolated intracranial lesions treated in the late window trials.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.00759.
Perfusion imaging criteria for patients in the late window
Multivariable analysis for the selected outcomes for late window patients with and without advance imaging
Forest plot showing late window adjusted effect for favorable clinical functionality (mRS 0–2) at 90 days in pre-specified groups. mRS, modified Rankin Scale; OR, odds ratio; CI, confidence interval; phet, heterogeneity P-value; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; IV, intravenous; ICA, internal carotid artery.
Forest plot showing early vs. late window adjusted effect for sICH and PH2 in pre-specified groups. sICH, symptomatic intracranial hemorrhage; PH2, parenchymal hematoma type 2; OR, odds ratio; CI, confidence interval; phet, heterogeneity P-value; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; IV, intravenous; ICA, internal carotid artery.
Notes
Funding statement
None
Conflicts of interest
Dr. Ortega-Gutierrez is a consultant for Medtronic and Stryker Neurovascular and received grants from NIH-NINDS (R01NS127114-01, R03NS126804), Stryker, Medtronics, Microvention, Penumbra, IschemiaView, Viz.ai, and Siemens. Dr. Malik reports no disclosures. Dr. Nguyen reports research support from Medtronic and SVIN. Dr. Hassan is a consultant/Speaker for Medtronic, Microvention, Stryker, Penumbra, Cerenovus, Genentech, GE Healthcare, Scientia, Balt, Viz.ai, Insera therapeutics, Proximie, NeuroVasc, NovaSignal, Vesalio, Rapid Medical, Imperative Care and Galaxy Therapeutics. He is Pi of COMPLETE study – Penumbra, LVO SYNCHRONISE – Viz.ai, Millipede Stroke Trial - Perfuze, RESCUE - ICAD - Medtronic. He is steering Committee/Publication committee member of: SELECT, DAWN, SELECT 2, EXPEDITE II, EMBOLISE, CLEAR, ENVI, DELPHI, DISTALS. And part of the DSMB - COMAND trial. Dr. Divani has received the following fundings: the University of New Mexico Center for Brain Recovery and Repair Center of Biomedical Research Excellence through Grant Number (NIH P20GM109089, Pilot PI), W81XWH-17-2-0053 (PI), 1R21NS130423-01 (PI). Dr. Siegler is a speaker bureau for AstraZeneca unrelated to the present work. Dr. Fifi is a consultant to Stryker, Cerenovus, Microvention, Viz.ai, and shareholder in Imperative Care. Dr. Petersen is supported by the NIH/NINDS (K23NS110980) and has received clinical research funding from Liminal Sciences. Dr. Galecio-Castillo reports research support from SVIN. Dr. Farooqui reports research funding from Microvention.
Author contribution
Conceptualization: SOG, MGC, MF. Study design: SOG, MGC, MF. Methodology: SOG, MGC, MF. Data collection: DQO, WT, SFZ, SYS, MOG, TB, RDL, KWS, MA, SSM, JS, WG, CT, JVS, ARC. Investigation: SOG, MGC, MF, AEH, MAJ, AAD, MR, MA, NHP, JTF, WRG, AMM, JWS, TNN, SS, AJY, GL, NJ, MM, DRY, TJ. Statistical analysis: MGC. Writing—original draft: SOG, MGC, MF. Writing—review & editing: AEH, MAJ, AAD, MR, MA, NHP, JTF, WRG, AMM, JWS, TNN, SS, AJY, GL, NJ, MM, DRY, TJ, DQO, WT, SFZ, SYS, MOG, TB, RDL, KWS, MA, SSM, JS, WG, CT, JVS, ARC. Approval of final manuscript: all authors.
Acknowledgements
We would like to acknowledge the following people for their contribution: Kathie Gonzales, CRA, University of Iowa Hospitals and Clinics; Stavros Matsoukas, Icahn School of Medicine at Mount Sinai.