The Impact of Genetically Proxied AMPK Activation, the Target of Metformin, on Functional Outcome Following Ischemic Stroke
Article information
Abstract
Background and Purpose
We performed a two-sample Mendelian randomization (MR) analysis to evaluate the causal effect of genetically proxied AMP-activated protein kinase (AMPK) activation, which is the target of metformin, on functional outcome following ischemic stroke onset.
Methods
A total of 44 AMPK-related variants associated with HbA1c (%) were used as instruments for AMPK activation. The primary outcome was the modified Rankin Scale (mRS) score at 3 months following the onset of ischemic stroke, evaluated as a dichotomous variable (3–6 vs. 0–2) and subsequently as an ordinal variable. Summary-level data for the 3-month mRS were obtained from the Genetics of Ischemic Stroke Functional Outcome network, including 6,165 patients with ischemic stroke. The inverse-variance weighted method was used to obtain causal estimates. The alternative MR methods were used for sensitivity analysis.
Results
Genetically predicted AMPK activation was significantly associated with lower odds of poor functional outcome (mRS 3–6 vs. 0–2, odds ratio [OR]: 0.06, 95% confidence interval [CI]: 0.01–0.49, P=0.009). This association was maintained when 3-month mRS was analyzed as an ordinal variable. Similar results were observed in the sensitivity analyses, and there was no evidence of pleiotropy.
Conclusion
This MR study provided evidence that AMPK activation by metformin may exert beneficial effects on functional outcome following ischemic stroke.
Introduction
Stroke is the second leading cause of death and the leading cause of disability worldwide [1]. From 1990 to 2019, the absolute number of deaths globally from ischemic stroke increased by 61.0%, and disability-adjusted life years due to ischemic stroke increased by 57.0% [1]. Given its huge socioeconomic burden, novel drug therapies are urgently needed for ischemic stroke.
Metformin, a well-established AMP-activated protein kinase (AMPK) activator, is the first-line antidiabetic drug to manage hyperglycemia in type 2 diabetes [2]. In addition to its antidiabetic effects, metformin may reduce the risk of cardiovascular disease and cancer in a manner that goes beyond glycemic control [3,4]. Plasma metformin concentrations in patients taking standard clinical doses of 1.5–2.0 g/d were only 5–30 μM [5]. Recently, Ma et al. [6] found that clinical doses of metformin (low doses of metformin) activated lysosomal AMPK through a novel PEN2-ATP6AP1-ATPase pathway, an AMP-independent mechanism. An experimental stroke study reported the neuroprotective effects of chronic metformin treatment on stroke outcomes, possibly through the modification of AMPK activity, which is a pharmacological target of metformin [7,8]. An observational study has shown that metformin-pretreated patients with diabetes who had acute stroke and underwent thrombolysis had better functional outcomes at 3 months following ischemic stroke than those not pretreated with metformin [9]. However, it is unclear whether these findings are clinically relevant because of the uncertain generalizability of preclinical studies to humans and the susceptibility of observational studies to confounding and reverse causation.
Mendelian randomization (MR) is a genetic epidemiological approach that uses genetic variants as proxies to study the causal effect of an exposure on an outcome; this approach is less vulnerable to confounding and reverse causality than traditional observational epidemiology [10,11]. MR has previously been used to study the causal effect of AMPK activation on the risk of cardiovascular disease and cancer [3]. Here, we performed this MR study using genetically proxied AMPK activation to study the AMPK pathway-dependent effects of metformin on functional outcome following ischemic stroke.
Methods
Study design
This study used a two-sample MR design that extracted summarized genetic association data for exposure and outcome from two independent non-overlapping populations, restricted to European-ancestry individuals to minimize bias due to population stratification.
Genetic instrument selection
We selected the genetic instruments for AMPK activation from a prior study by Luo et al. [3]. Specifically, Luo et al. [3] constructed the genetic instruments using the following approaches: (1) single nucleotide polymorphisms (SNPs) within 1 MB pairs upstream and downstream of genes that encode AMPK subunits; (2) SNPs associated with HbA1c (%) (P≤0.05) in the Meta-Analyses of Glucose and Insulin-Related Traits Consortium (MAGIC) European database including 123,665 participants without diabetes [12]; (3) SNPs were in low linkage disequilibrium (LD, r2<0.3); and (4) SNPs were also associated with HbA1c (P≤0.05) in the UK Biobank including 391,199 individuals. Based on the above approaches, Luo et al. [3] identified 44 SNPs as instruments for AMPK activation. The instruments for AMPK activation have been validated in positive control outcomes of HbA1c and type 2 diabetes [3]. We used the 44 SNPs (r2<0.3) as genetic instruments for AMPK activation in the primary analyses (Supplementary Table 1). This relatively lenient LD threshold (r2<0.3) can increase the proportion of variance explained and thus improve statistical power. In the sensitivity analyses, we restricted our instrument selection to a more stringent LD correlation threshold (r2<0.1).
Study outcome
The primary outcome was the modified Rankin Scale (mRS) score at 3 months following the onset of ischemic stroke, evaluated as a dichotomous variable (mRS 3–6 vs. 0–2) and subsequently as an ordinal variable (ordinal mRS). Poor functional outcomes were defined by a 3-month mRS score of 3–6. Genome-wide association study (GWAS) summary-level data for 3-month mRS were extracted from the Genetics of Ischaemic Stroke Functional Outcome (GISCOME) network, which includes 6,165 ischemic stroke patients of European ancestry [13]. In the present study, we used two formulations of 3-month mRS: dichotomous mRS (mRS 3–6 vs. 0–2) and ordinal mRS. In the GISCOME study, 2,335 patients (37.9%) suffered poor functional outcome (mRS 3–6). Given that metformin has been reported to exert protective effects even before ischemic stroke onset [9], we used the GWAS results without adjusting for baseline National Institutes of Health Stroke Scale (NIHSS), and the GWAS results were adjusted for age, sex, and principal components. In the sensitivity analyses, we also used the GWAS results adjusted for NIHSS, age, sex, and principal components.
Evaluating the association of genetically predicted AMPK activation with risk of ischemic stroke using MR
An important issue of MR in case-only studies is collider bias [14]. To evaluate whether the observed effects of AMPK activation on functional outcome following ischemic stroke were due to collider bias, we evaluated the causal effect of genetically predicted AMPK activation on the risk of ischemic stroke. In the absence of collider bias, the association between genetically proxied AMPK activation and the risk of ischemic stroke should be insignificant. Summary data for ischemic stroke were extracted from the MEGASTROKE consortium, which includes 34,217 patients and 406,111 controls of European descent [15].
Evaluating the impact of genetically predicted HbA1c on functional outcome following ischemic stroke using MR
To investigate whether the effect of AMPK activation on functional outcomes following ischemic stroke occurred via HbA1c lowering or other mechanisms, we evaluated the association between genetically predicted HbA1c levels and functional outcomes following ischemic stroke. We obtained 99 independent SNPs robustly associated with HbA1c (%) from MAGIC (P<5×10-8, r2<0.01) (Supplementary Table 2) [16], and found no overlap between the 99 HbA1c and 44 AMPK SNPs.
Ethics and patient consent
All data were obtained from GWAS studies with ethical review board approval, and all participants provided informed consent.
Statistical analyses
We calculated individual MR estimates and their standard errors using the Wald ratio and the delta method, respectively [17]. The MR estimates of genetically proxied AMPK activation and outcomes were evaluated by pooling individual MR estimates using the fixed effects inverse-variance weighted (IVW) method [17]. The heterogeneity across individual SNPs was assessed using Cochran’s Q test (statistical significance set at P<0.05). To check the robustness of the findings, we used several MR methods that are more robust to pleiotropy. The weighted median estimator requires that ≥50% of the weight is obtained from valid instruments [18]. The MR–robust adjusted profile score (MR-RAPS) with the Huber loss function models were used to assess the pleiotropic effects of SNPs using a random-effects distribution [19]. The MR–Egger regression produced MR estimates adjusted unbalanced pleiotropy, and the MR–Egger intercept test indicated whether there was unbalanced pleiotropy [20]. However, due to a low statistical power, MR–Egger estimate generally had a lower precision than the IVW estimate [20]. Finally, the MR pleiotropy residual sum and outlier (MR-PRESSO) test was used to detect pleiotropic outliers [21].
Odds ratios (ORs) and 95% confidence intervals (CIs) were scaled per 1% decrease in HbA1c. All statistical analyses were performed with R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), using the MendelianRandomization [22], mr.raps [19], MR-PRESSO [21], and TwoSampleMR packages [23].
Data availability statement
This study uses publicly available summary-level data. Summary-level data for ischemic stroke and 3-month mRS scores were obtained from the MEGASTROKE consortium and the Cerebrovascular Disease Knowledge Portal created by the International Stroke Genetics Consortium, respectively. The genetic variants used as instruments are provided in Supplementary Table 1 and 2.
Results
According to the IVW analysis, a genetically predicted reduction in HbA1c (%) instrumented by 44 AMPK variants was significantly associated with decreased odds of poor functional outcomes (mRS 3–6 vs. 0–2, OR: 0.06, 95% CI: 0.01–0.49, P=0.009) (Table 1). This association was maintained when 3-month mRS was analyzed as an ordinal variable (OR: 0.08, 95% CI: 0.01–0.47, P=0.005). The effects remained directionally consistent in MR-RAPS, weighted median, and MR–Egger estimates (Table 1), although with wider 95% CIs, owing to low statistical power. In addition, similar results were obtained in the analyses restricted to instrument selection with a lower LD threshold (r2<0.1) (Table 1) and in the analyses based on the 3-month mRS GWAS with adjustment for NIHSS (Supplementary Table 3). Cochran’s Q test and MR–Egger intercepts indicated no evidence of pleiotropy (P>0.27). Similarly, the MR-PRESSO test did not detect any potential outliers.
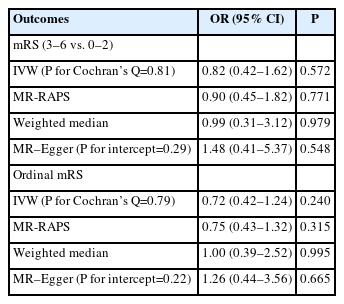
In all MR methods, we did not find robust evidence of genetically predicted reduction in HbA1c (%) instrumented by 44 AMPK variants being associated with risk of ischemic stroke (IVW OR: 0.80, 95% CI: 0.48–1.35, P=0.412) (Table 2), supporting the contention that their associations with functional outcome may not be attributed to collider bias. In addition, using 99 HbA1c instruments, we found no evidence that genetically predicted reduction in HbA1c (%) was associated with the odds of poor functional outcome (mRS 3–6 vs. 0–2, IVW OR: 0.82, 95% CI: 0.42– 1.62, P=0.572) (Table 3) and higher 3-month disability as quantified by ordinal mRS (IVW OR: 0.72, 95% CI: 0.42–1.24, P=0.240) (Table 3), which suggests that the protective effect of metformin, via AMPK pathways, on functional outcome following ischemic stroke is likely to occur through a glycemic-independent mechanism. In all analyses, we found no evidence of pleiotropy, as determined by Cochran’s Q test and MR–Egger intercepts (P>0.05) (Tables 2 and 3). Similarly, MR-PRESSO tests did not detect any potential outliers.
Discussion
This is the first MR study to investigate the causal effects of metformin based on AMPK variants on functional outcome following ischemic stroke. Our findings suggest that AMPK activation by metformin may exert beneficial effects on functional outcome following ischemic stroke. In addition, our findings support the protective effect of metformin via the AMPK pathway likely to occur via a glycemic-independent mechanism.
Only one observational study in the European population has focused on evaluating the effect of pre-stroke metformin use on stroke severity and functional outcome; this study included 1,919 patients with ischemic stroke and type 2 diabetes who underwent intravenous thrombolysis [9]. Westphal et al. [9] found that metformin-pretreated patients presented with less severe stroke on admission and better functional outcomes at 3 months than those not pretreated with metformin. Furthermore, the researchers found no evidence of differences in HbA1c levels between metformin-pretreated patients and those not pretreated with metformin, supporting the protective effect of pre-stroke metformin use on post-stroke outcomes that goes beyond glycemic control. This MR study differs from the previous observational study by Westphal et al. [9] in two respects. First, our MR study evaluated the effects of genetically proxied AMPK activation in a non-diabetic population; however, the previous study was conducted in patients with diabetes. Second, this observational study was influenced by unmeasured confounding factors. Conversely, because genetic variants are randomly assigned at conception, the MR is less influenced by confounding factors.
MR estimates represent the effects of lifetime exposure; thus, genetically proxied AMPK activation reflects long-term AMPK activation by chronic pre-stroke metformin use. An experimental stroke study also showed that long-term AMPK activation by chronic pre-stroke metformin use exerts a protective effect on stroke outcome [8]. Li et al. [8] evaluated the effect of chronic prestroke metformin treatment on experimental stroke and found that chronic pre-stroke metformin treatment reduced stroke-induced lactate formation. Long-term AMPK activation by chronic metformin treatment may enhance lactate levels in the intact brain under sublethal metabolic stress, thereby making the brain less susceptible to subsequent injury [8].
The key strength of this study is the use of genetic variants within genes that encode AMPK subunits for evaluating the potential effect of proxied AMPK activation by metformin use on post-stroke outcomes, which should minimize confounding and time-related biases (e.g., reverse causation).
This study has several limitations. First, our MR study may only evaluate the effect of metformin on functional outcomes following ischemic stroke via AMPK activation pathways. Further studies are needed to assess whether there are AMPK-independent pathways involved in the effect of metformin on post-stroke outcome. Second, the MR estimates are the effects of lifetime exposure, whereas randomized controlled trials (RCTs) evaluate short-term pharmacological treatment; thus, further RCTs are warranted to verify the protective effect of metformin on post-stroke outcomes. Third, an important issue of applying MR in case-only studies is collider bias [14]. In this study, if genetically proxied AMPK activation was associated with ischemic stroke onset, the genetic instruments may be associated with risk factors for onset; thus, the association between the genetic instruments and functional outcome following ischemic stroke may be vulnerable to confounding by these factors. However, we found that genetically proxied AMPK activation was not associated with the onset of ischemic stroke, suggesting that collider bias was unlikely to have affected our findings. Fourth, the threshold used for the AMPK variant selection was lower than the conventional genome-wide significance threshold. Nevertheless, the association of variants with HbA1c was verified in two independent studies (MAGIC and UK Biobank), which reduced the possibility of false positives. In addition, 44 AMPK variants were validated in the positive control outcomes of HbA1c and type 2 diabetes. Fifth, our MR study only estimated linear causal effects, and further nonlinear MR studies are needed to study the dose-response effect of metformin on post-stroke outcomes. Sixth, the effects of metformin on post-stroke outcomes for different ischemic stroke subtypes are unclear, and further studies are warranted. Seventh, there is no evidence of AMPK activation in humans at the clinical dose of metformin. Finally, our study was restricted to the European population, which limits the generalizability of our findings to other populations.
Conclusions
This MR study provides evidence that long-term AMPK activation by metformin use may exert beneficial effects on functional outcome following ischemic stroke. Further research is needed to verify these protective effects on functional outcomes after ischemic stroke.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2022.03230.
Fourty-four AMPK variants associated with HbA1c in the MAGIC, restricted to participants of European descent
Ninety-nine variants associated with HbA1c in the MAGIC, restricted to participants of European descent
Associations of genetically proxied AMPK activation with functional outcome after ischemic stroke based on the 3-month GWAS results with adjustment for NIHSS
Notes
Funding statement
Mengmeng Wang received funding from the Changzhou Sci&Tech Program (Grant No. CJ20210084), and the Young Talent Development Plan of Changzhou Health Commission (No. CZQM2022001). Marios K. Georgakis was funded by a Walter-Benjamin fellowship from the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG], GZ: GE 3461/1-1), the FöFoLe program of LMU Munich (FöFoLe-Forschungsprojekt Reg.-Nr. 1120), DFG Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198), and a Research Grant from the Fritz-Thyssen Foundation (Ref. 10.22.2.024MN). Ville Karhunen was funded by the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 848158 (EarlyCause) and Academy of Finland Project 326291.
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: MW, ZZ, MKG. Study design: all authors. Methodology: MW. Data collection: MW. Investigation: MW. Statistical analysis: MW. Writing—original draft: MW. Writing— review & editing: VK, DL. Funding acquisition: MW, MKG, VK. Approval of final manuscript: all authors.
Acknowledgements
The authors wish to thank the Genetics of Ischaemic Stroke Functional Outcome (GISCOME) network, ISGC Cerebrovascular Disease Knowledge Portal, MAGIC, and MEGASTROKE consortium for making their data publicly available. All the authors of the MEGASTROKE consortium are listed at http://www.megastroke.org/authors.html. The MEGASTROKE project received funding from source specified at https://www.megastroke.org/acknowledgements.html.