Borderzone Infarcts and Recurrent Cerebrovascular Events in Symptomatic Intracranial Arterial Stenosis: A Systematic Review and Meta-Analysis
Article information
Abstract
Background and Purpose
Intracranial arterial stenosis (ICAS)-related stroke occurs due to three primary mechanisms with distinct infarct patterns: (1) borderzone infarcts (BZI) due to impaired distal perfusion, (2) territorial infarcts due to distal plaque/thrombus embolization, and (3) plaque progression occluding perforators. The objective of the systematic review is to determine whether BZI secondary to ICAS is associated with a higher risk of recurrent stroke or neurological deterioration.
Methods
As part of this registered systematic review (CRD42021265230), a comprehensive search was performed to identify relevant papers and conference abstracts (with ≥20 patients) reporting initial infarct patterns and recurrence rates in patients with symptomatic ICAS. Subgroup analyses were performed for studies including any BZI versus isolated BZI and those excluding posterior circulation stroke. The study outcome included neurological deterioration or recurrent stroke during follow-up. For all outcome events, corresponding risk ratios (RRs) and 95% confidence intervals (95% CI) were calculated.
Results
A literature search yielded 4,478 records with 32 selected during the title/abstract triage for full text; 11 met inclusion criteria and 8 studies were included in the analysis (n=1,219 patients; 341 with BZI). The meta-analysis demonstrated that the RR of outcome in the BZI group compared to the no BZI group was 2.10 (95% CI 1.52–2.90). Limiting the analysis to studies including any BZI, the RR was 2.10 (95% CI 1.38–3.18). For isolated BZI, RR was 2.59 (95% CI 1.24–5.41). RR was 2.96 (95% CI 1.71–5.12) for studies only including anterior circulation stroke patients.
Conclusion
This systematic review and meta-analysis suggests that the presence of BZI secondary to ICAS may be an imaging biomarker that predicts neurological deterioration and/or stroke recurrence.
Introduction
Intracranial atherosclerotic disease (ICAS) is a common cause of stroke worldwide [1], accounting for up to 50% of ischemic strokes in China [2] and nearly 10% of ischemic strokes in the United States [3] and Europe [4]. It is associated with a high risk of recurrence in medically treated patients, particularly early after the initial event [5-9]. ICAS causes ischemic stroke either by distal embolization, perforator disease, and/or by impaired blood flow/perfusion across a highly stenotic artery [1,10-14]. Studies have shown that in medically treated patients with symptomatic ICAS, certain biomarkers of impaired distal blood flow or perfusion are associated with increased stroke recurrence [15-19].
Borderzone infarct (BZI) pattern indirectly implies blood flow impairment [20,21] and perfusion delay [19,22] distal to the arterial stenosis. Several studies have shown that BZI is associated with an increased risk of recurrence in patients with symptomatic ICAS related to possible hemodynamic impairment [18,23-26]. Such studies, however, were small, observational, underpowered, mostly single-center based and were subject to confounding bias.
We performed a systematic review and meta-analysis to evaluate the hypothesis that BZI is associated with a higher risk of recurrent ischemic stroke or neurological deterioration compared to other ICAS-related infarct patterns such a perforator disease or artery-to-artery embolism.
Methods
Study design
This is a systematic review and meta-analysis registered in International Prospective Register of Systemic Reviews (PROSPERO, CRD42021265230) and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (Supplementary Table 1). As this study used published de-identified data, ethics approval was waived by the Lifespan Institutional Review Board. Data from this study is available upon request to the corresponding author.
Inclusion and exclusion criteria
We included retrospective or prospective observational studies (manuscripts or conference abstracts) of patients 18 years or older with an ischemic stroke in the setting of ICAS. Studies with fewer than 20 patients and those with full texts in languages other than English were excluded during the initial screening. Duplicate studies and those not reporting the association between infarct pattern and our study outcome were excluded during the second stage of study selection.
Primary predictor
The primary predictor was the presence of BZI, either in isolation (isolated BZI) or along with other infarct patterns.
Outcome
The outcome was recurrent cerebrovascular events during follow-up defined as new or worsening neurological symptoms caused by: (1) new distinct infarct (recurrent ischemic stroke) or (2) extension of the existing infarct (neurological deterioration). This outcome does not include transient ischemic attack [27].
Search criteria
A comprehensive search was performed by a health science librarian (RM) using combinations of vocabulary, title, and abstract keywords in MEDLINE (via PubMed), Scopus (Elsevier), Cochrane Library, and Web of Science Core Collection (Clarivate Analytics). The search was performed from the conception of the above databases until October 18, 2021, with an updated search on March 15, 2022. An additional targeted search of conference abstracts for the International Stroke Conference, European Stroke Organization Conference, and the American Academy of Neurology was performed using the Web of Science’s conference search field on June 13, 2022. The search was conducted using the name of the conference in combination with the term “intracranial stenosis” in all fields. The complete search strategy for all databases is presented in Supplementary Table 2.
Screening
The search results were then imported into Abstrackr (http://abstrackr.cebm.brown.edu) [28], which each study screened for eligibility independently by two of seven reviewers (SY, AS, BM, SKB, CO, DC, and FF). Disagreements were resolved by a third reviewer. Potentially eligible abstracts were then screened in detail using the full text if available, and for excluded abstracts, the reasons for exclusion were recorded.
Data extraction
Extracted data included details of each study: age, sex, followup duration, BZI definition, outcome definition, ischemic stroke location (anterior circulation vs. posterior circulation), and outcome rates between the two groups (BZI vs. non-BZI). Data was extracted independently by two of four investigators (EDG, LS, AD, and SD), with disagreements resolved by a third investigator followed by consensus between the three investigators. Due to missing raw data in one study [25], the corresponding author was contacted and provided raw data for inclusion in the data synthesis. In addition, further analysis of data from one study [29] was done by authors (SY and AK).
Risk of bias assessment
Risk of bias was assessed (by MA and LP) for all studies (all observational) using the Risk of Bias in Nonrandomized Studies (ROBINS-I) tool [30]. The ROBINS-I tool assesses bias based on confounding, participant selection, classification of intervention, deviations from intervention, missing data, outcome measurement, and selection of the reported results.
Publication bias assessment
Publication bias was assessed in analyses including at least 10 studies [31] using both the Egger’s test [32] and inspection of the funnel plot.
Data synthesis
We summarized the results both narratively and quantitatively when possible. We calculated risk ratios as the data allowed. When at least two studies were sufficiently similar, a pairwise meta-analysis was performed using a random effects model. The appropriateness of the meta-analysis was determined based on clinical/methodological (characteristics of the studies and definitions of predictors/outcomes) as well as statistical heterogeneity using I2 values using the Cochrane Handbook [33] (0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; and 75% to 100%: considerable heterogeneity). We conducted subgroup analyses stratified based on (1) definition of BZI (any BZI vs. isolated BZI), (2) location of ICAS (anterior circulation vs. anterior and posterior circulation), (3) definition of outcome (neurological deterioration, recurrent ischemic stroke, or both), and (4) follow-up duration (≤90 days vs. >90 days). These analyses were prespecified in our meta-analysis protocol.
Results
Search and screening results
Among the 4,478 records generated by the initial search, 32 abstracts were selected and after detailed review, 10 met the inclusion criteria and an additional conference abstract was selected yielding 11 studies. Reasons for exclusion included no information on BZI (12 records), no information on association between BZI and study outcome (5 records), no information on study outcome (1 record), and duplicate records (4 records) (Figure 1).
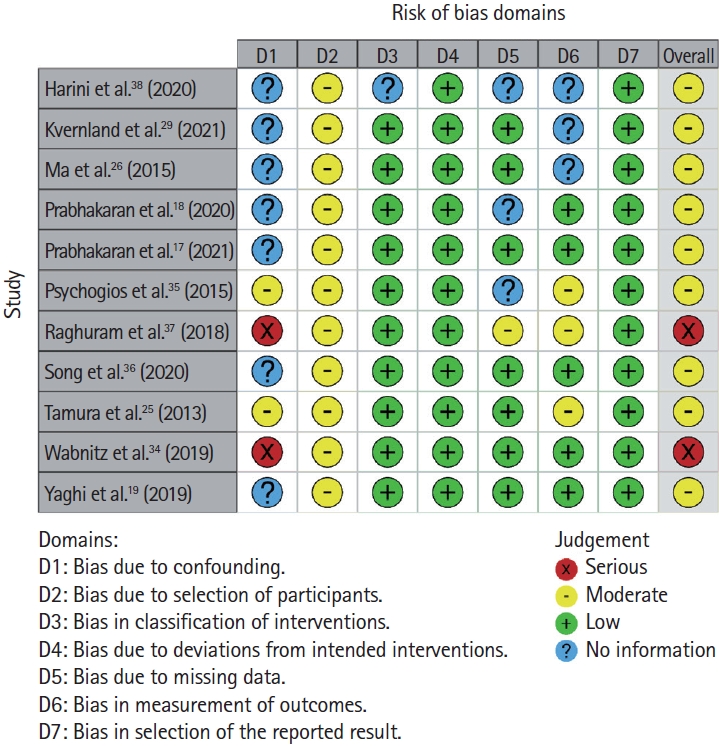
Risk of bias assessment
The risk of bias assessment is shown in Figure 2. All retrospective studies had at least moderate bias in most of the seven domains, the prospective study and those with retrospective analysis of prospective studies had low to moderate bias in most domains.
Risk of publication bias assessment
Since all analyses included <10 studies, publication bias using Egger’s test was not performed [31].
Characteristics of studies and results of systematic review
The characteristics of included studies are shown in Table 1. Among the 11 studies included, 1 was prospective [17], 3 were retrospective analyses of prospectively collected data [18,26,34], and 7 were retrospective studies [19,25,29,35-38]. The primary outcome was early neruological deteroriation in 2 studies [25,26], and it was one of the few primary outcomes reported in 2 other studies [19,29]. The definition of BZI was any infarct involving the borderzone area in 5 studies [17-19,25,29] or infarct(s) isolated to the borderzone territory in 4 studies [34,35,37,38], and unclear in 2 studies [26,36]. The outcome was captured in-hospital in 2 studies [25,26], within 90 days from index event in 5 studies [17-19,29,38], and beyond 90 days in 4 studies [34-37]. All studies except 1 study [38], demonstarted an association between BZI and: (1) recurrent stroke [18,19,29,34-37], (2) recurrent infarct [17], or (3) neurological deterioration [25,26].
Association between borderzone infarct and recurrent cerebrovascular events
Recurrent cerebrovascular events were assessed in 8 studies [18,19,25,26,29,34,35,37] and 3 studies were not included in the data synthesis (1 study [17] due to outcome being recurrent infarct and 2 studies [36,38] due to not providing raw data for event rates across the two groups). The 8 studies included a total of 1,219 patients with 341 BZI. The weighted proportion of patients with BZI was 28.0% (25.5%–30.6%). When compared to non-BZI, BZI was associated with a higher risk of recurrent cerebrovascular events (RR 2.10, 95% CI 1.52–2.90, P for Cochran Q: <0.01, I2=38.68%) (Figure 3A).
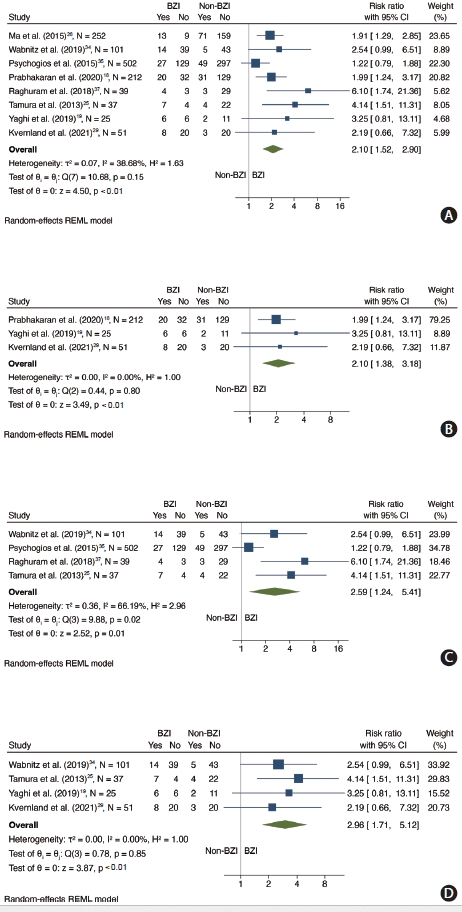
Forest plot showing association between BZI and recurrent cerebrovascular events. (A) Association between BZI and recurrent cerebrovascular events. (B) Association between any BZI and recurrent cerebrovascular events. (C) Association between isolated BZI and recurrent cerebrovascular events. (D) Association between BZI and recurrent cerebrovascular cerebrovascular events in patients with anterior circulation. BZI, borderzone infarct; CI, confidence interval; REML, restricted maximum likelihood.
Subgroup analyses by definition of borderzone infarct
In a subgroup analysis limited to studies assessing any BZI as the predictor (n=325 patients, 4 studies) [18,19,25,29], there was an association between any BZI and recurrent cerebrovascular events (RR 2.10, 95% CI 1.38–3.18, P for Cochran Q: <0.01, I2=0%) (Figure 3B). Furthermore, when the analysis was limited to studies assessing isolated BZI as the predictor (n=679 patients, 4 studies) [25,34,35,37], the association between BZI and recurrent cerebrovascular events was statistically significant (RR 2.59, 95% CI 1.24– 5.41, P for Cochran Q: 0.02) but there was substantial statistical heterogeneity (I2=66.19%) across included studies (Figure 3C).
Subgroup analyses by outcome definition and timing
In a subgroup analysis limited to studies whose outcome was defined as recurrent ischemic stroke during follow-up (n=854 patients, 4 studies) [18,34,35,37], we found an association between BZI and recurrent ischemic stroke (RR 2.03, 95% CI 1.19–3.47, P for Cochran Q: 0.01, I2=60.95%) (Figure 4A). Furthermore, in a subgroup analysis limiting studies to those where the outcome encompassed neurological detrioration [19,29] or was limited to in-hospital neurological deterioration outcome [25,26] (n=365 patients, 4 studies), there was an association between BZI and recurrent ischemic stroke (RR 2.27, 95% CI 1.52–3.38, P for Cochran Q: <0.01, I2=8.95%) (Figure 4B). The association between BZI and recurrent cerebrovascular events was present in studies assessing in-hospital outcomes (n=289 patients, 2 studies) [25,26] (RR 2.44, 95% CI 1.21–4.91, P for Cochran Q: 0.01, I2=48.68%) (Figure 4C) and short term recurrent cerebrovascular events (within 90 days) (n=288 patients, 3 studies [18,19,29] (RR 2.10, 95% CI 1.38–3.18, P for Cochran Q: <0.01, I2=0%) (Figure 4D). There was no association between BZI and long-term outcome (>90 days) [34,35] (n=603 patients, 2 studies) (RR 1.55, 95% CI 0.79–3.04, P for Cochran Q: 0.20, I2=47.47%) (Figure 4E).
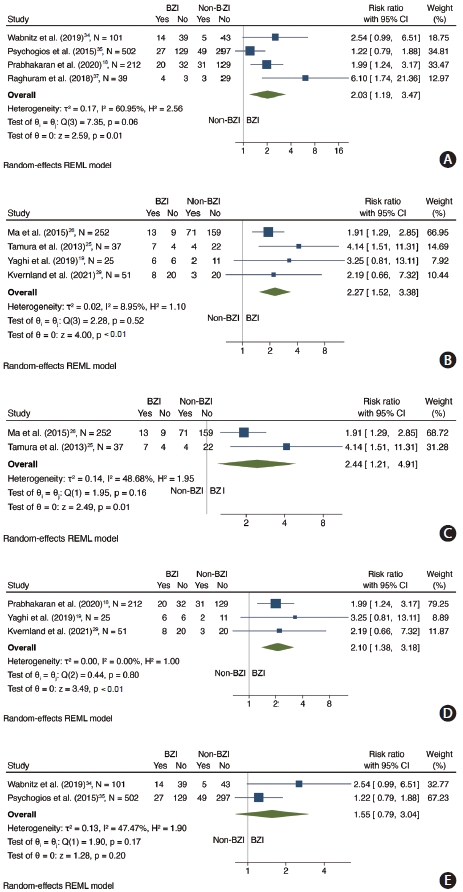
Forest plot showing subgroup analyses by outcome definition and timing. (A) Association between BZI and recurrent ischemic stroke. (B) Association between BZI and recurrent cerebrovascular events in studies whose outcome included or was limited to in-hospital neurological deterioration outcome. (C) Association between BZI and in-hospital recurrent cerebrovascular events. (D) Association between BZI and recurrent cerebrovascular events within 90 days. (E) Association between BZI any recurrent cerebrovascular events in studies with >90-day follow-up. BZI, borderzone infarct; CI, confidence interval; REML, restricted maximum likelihood.
Subgroup analysis limiting to studies including anterior circulation stroke
In a subgroup analysis limited to studies including only anterior circulation stroke (n=214 patients, 4 studies) [19,25,29,34], there was an association between BZI and recurrent ischemic stroke (RR 2.96, 95% CI 1.71–5.12, P for Cochran Q: <0.01, I2=0%) (Figure 3D).
Sensitivity analysis
Sensitivity analysis stratifying by continent of published study yielded similar findings (Supplementary Figure 1).
Discussion
Summary of findings
This systematic review and meta-analysis suggests that BZI may be associated with an increased risk of recurrent cerebrovascular events compared with other infarct patterns. The association seemed to be more pronounced in studies assessing early as opposed to long-term recurrent cerebrovascular events. Furthermore, the effect size was larger when limiting the studies to anterior circulation only and to those defining BZI as the presence of one or more infarct involving the borderzone territory even when occurring in conjunction with non-BZI.
Implications for clinical practice
The finding that the presence of a BZI is associated with recurrent cerebrovascular events, particularly in the first 90 days, is noteworthy. In patients with ICAS, BZI indirectly implies impaired blood flow [20,21], impaired clearance of emboli [39], or perfusion delay [19,22] across a stenosed artery. Although medical treatment can help stabilize atherosclerotic plaques and reduce the risk of thrombosis and embolization, it is unlikely to improve blood flow/perfusion in the affected territory in the acute setting. Thus, medical treatment may not be effective in preventing early recurrent stroke or neurological deterioration in patients with impaired distal blood flow/perfusion.
Endovascular trials of ICAS such as the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) [7] and the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) [8] trials raised safety concerns with regard to endovascular treatment. These trials selected patients based on the degree of intracranial stenosis rather than based on blood flow/perfusion status. In fact, a post hoc analysis of SAMMPRIS [34] not only showed that the presence of BZI in medically treated patients was associated with increased recurrence risk but also that patients with BZI had fewer events when treated with stenting versus medical treatment. More recently, the China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) trial showed randomized patients with symptomatic 70%–99% ICAS excluding those with perforator infarct [40]. CASSISS showed no significant difference in recurrence risk between medical treatment and endovascular treatment [40]. Similar to VISSIT and SAMMPRIS, CASSISS was not limited to patients with impaired perfusion/flow.
Therefore, given the high risk of recurrence in patients with symptomatic ICAS and BZI and a potential mechanistic benefit from reperfusion, studies testing the safety and efficacy of angioplasty with or without stenting in patients with symptomatic ICAS and BZI are needed.
Limitations
There are several limitations to our study. First, we restricted studies to the English language. Given that ICAS is more prevalent in Asia, we may have missed relevant studies published in languages other than English. Second, our assessment of the studies suggested a high risk of bias, which could have impacted our results. Third, the definition of BZI and outcomes varied across the studies. However, our findings were largely consistent in various subgroups stratified based on various definitions of BZI and outcomes, suggesting that this limitation is unlikely to have impacted our findings. Fourth, we did not examine time-to-event data since this information was unavailable in the included studies. Fifth, we pooled studies with different design (retrospective vs. prospective, single-center vs. multi-center). Sixth, given the small number of studies included, we are unable to rule out the potential for publication bias. Finally, other factors that can contribute to recurrence such as plaque stability and lesion multiplicity were not captured in our study. Thus comprehensive meta-analyses addressing these issues can help understand the pathophysiology of recurrent cerebrovascular events in ICAS.
Conclusions
In this systematic review and meta-analysis, in patients with symptomatic ICAS, BZI is associated with recurrent cerebrovascular events, particularly in the early period after the index event. Given the limitations of the identified evidence, our findings should be interpreted with caution pending confirmation by prospective studies with core lab adjudication.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2023.00185.
PRISMA 2020 Checklist
Database search strategies
Forest plot showing sensitivity analysis stratifying by continent. BZI, borderzone infarct; CI, confidence interval; REML, restricted maximum likelihood.
Notes
Funding statement
None
Conflicts of interest
The authors have no financial conflicts of interest.
Author contribution
Conceptualization: SD, LS, RJM, EG, BMG, AD, EM, SY. Study design: SD, LS, RJM, EG, AD, EM, SY. Methodology: SD, LS, RJM, EG, AD, EM, SY. Data collection: SD, AS, FHF, DC, BM, SKB, CO, MA, AK, LP. Investigation: SD, LS, RJM, EG, AD, EG, AD, EM, SY. Statistical analysis: LS, LP, SY. Writing—original draft: SD, LS, RJM, SY. Writing—review & editing: SD, JES, TN, EG, BMG, KF, PK, SP, DSL, JGR, ADH, GT, SY. Approval of final manuscript: all authors.