Impact of Multiphase Computed Tomography Angiography for Endovascular Treatment Decision-Making on Outcomes in Patients with Acute Ischemic Stroke
Article information
Abstract
Background and Purpose
Various imaging paradigms are used for endovascular treatment (EVT) decision-making and outcome estimation in acute ischemic stroke (AIS). We aim to compare how these imaging paradigms perform for EVT patient selection and outcome estimation.
Methods
Prospective multi-center cohort study of patients with AIS symptoms with multi-phase computed tomography angiography (mCTA) and computed tomography perfusion (CTP) baseline imaging. mCTA-based EVT-eligibility was defined as presence of large vessel occlusion (LVO) and moderate-to-good collaterals on mCTA. CTP-based eligibility was defined as presence of LVO, ischemic core (defined on relative cerebral blood flow, absolute cerebral blood flow, and cerebral blood volume maps) <70 mL, mismatch-ratio >1.8, absolute mismatch >15 mL. EVT-eligibility and adjusted rates of good outcome (modified Rankin Scale 0–2) based on these imaging paradigms were compared.
Results
Of 289/464 patients with LVO, 263 (91%) were EVT-eligible by mCTA-criteria versus 63 (22%), 19 (7%) and 103 (36%) by rCBF, aCBF, and CBV-CTP-criteria. CTP and mCTA-criteria were discordant in 40% to 53%. Estimated outcomes were best in patients who met both mCTA and CTP eligibility-criteria and were treated with EVT (62% to 87% good outcome). Patients eligible for EVT by mCTA-criteria and not by CTP-criteria receiving EVT achieved good outcome rates of 53% to 57%. Few patients met CTP-criteria and not mCTA-criteria for EVT.
Conclusions
Simpler imaging selection criteria that rely on little else than detection of the occluded blood vessel may be more sensitive and less specific, thus resulting in more patients being offered EVT and arguably benefiting from it.
Introduction
The vanguard trials that established efficacy of endovascular treatment (EVT) in patients with acute ischemic stroke (AIS) used various imaging criteria for patient selection. These ranged from simple paradigms like non-contrast head computed tomography (NCCT) and single-phase computed tomography angiography (CTA) in the Multicenter Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) [1] and the EndoVascular Treatment With Stent-retriever and/or Thromboaspiration vs. Best Medical Therapy in Acute Ischemic Stroke (RESILIENT) trial (NCT02216643) to collateral imaging using multi-phase computed tomography angiography (mCTA) in the Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE) trial [2] to multimodal magnetic resonance imaging (MRI) in the Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke (THRACE) trial. Computed tomography perfusion (CTP) was used exclusively in the Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial (EXTEND IA) trial [3], a mixture of advanced imaging was used in the Solitaire™ With the Intention For Thrombectomy as PRIMary Endovascular Treatment (SWIFT PRIME) trial [4] (some mCTA and some CTP) and the late window Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) [5] and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE-3) [6] trials had NCCT and CTP paradigms. Each approach has advantages and drawbacks but the lack of standardization of imaging paradigms globally results in misunderstanding about what types of patients are actually being enrolled into studies. This makes comparison of patient populations and treatment effects difficult, but it also constitutes an opportunity to compare different imaging paradigms and try to find an optimal imaging approach; one that provides just enough information for treatment decision-making and outcome prediction, without delaying or compromising treatment by either obtaining unnecessary information or excluding patients who may have benefited from treatment.
Of all the imaging paradigms in use in patients with acute stroke, NCCT with single phase CTA is the simplest and arguably the fastest, but reliability of assessment of the extent of ischemia is low, particularly among non-experts. Pial collateral status assessment has high specificity if the collaterals are good on a single phase CTA but poor collateral filling could be a false result due to delay in timing of the contrast bolus and consequent arterial filling [7]. Both CTP and mCTA provide time-resolved images that try to address the issue of mistimed bolus contrast influencing assessment of contrast enhanced computed tomography (CT) in patients with acute stroke [8,9]. In CTP, the brain is continuously scanned over 45 to 90 seconds, while in mCTA, three scan cycles are performed over 16 to 20 seconds [8]. Due to its higher temporal resolution, the information content in CTP images is higher when compared to mCTA, but this comes at the cost of lower spatial resolution, higher motion susceptibility and requirement for algorithm based image postprocessing.
Using a population of suspected AIS patients presenting within 12 hours of last known well, we compare CTP imaging for EVT decision-making to mCTA-based imaging to examine how much advanced imaging information is needed for patient selection and outcome prediction at a population level.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the local Institutional Review Board. The study protocol was registered on clinicaltrials.org (NCT02184936). The study was conducted according to the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. Patient consent was obtained prior to enrolment.
Data availability
Anonymized data will be made available by the corresponding author upon reasonable request.
Study participants
The Precise and Rapid Assessment of Collaterals Multi-phase CTA in the Triage of Patients With Acute Ischemic Stroke for IA Therapy (Prove-IT) study was a prospective multi-center cohort study that enrolled 595 patients who presented with AIS symptoms. Patients were included if they presented to the emergency department with symptoms consistent with AIS, were older than 18 years, and mCTA and CTP were both performed within 12 hours of symptom onset and before recanalization therapy. Exclusion criteria were intracranial hemorrhage at baseline NCCT, previous sizeable stroke in the ipsilateral hemisphere, modified Rankin Scale (mRS) >2 at baseline, estimated creatinine clearance <60 mL/min, contrast material allergy, or other contraindications for iodinated contrast and estimated life expectancy <1 year [8]. The enrollment period was July 2012 to October 2016.
Imaging protocol
NCCT and mCTA
NCCT with 5 mm slice thickness was obtained, followed by a CTA with arch to vertex coverage (CTA Head and Neck; conventional single phase CTA). This first phase was followed by skull base to vertex coverage for the second (peak venous) and third (late venous) phase of a mCTA acquisition. Detailed mCTA acquisition parameters have been published previously [8]. Axial images with 1 mm overlap and multiplanar axial, coronal and sagittal reconstructions with 3 mm thickness, 1 mm intervals, and 1 mm overlap for the first phase were obtained, along with axial minimum intensity projections for all three phases with 24 mm thickness and 4 mm intervals.
CTP
Forty-five milliliter of iodinated contrast agent were injected at a rate of 4.5 mL/sec followed by a 40 mL saline bolus injected at a rate of 6 mL/sec. Image acquisition started 5 seconds after contrast injection and 24 passes over 66 seconds were performed with 5 mm section thickness and a cranio-caudal coverage of 8 cm. CTP source data were centrally processed in a non-acute setting for each study using a delay-insensitive deconvolution software (CTP 4D; GE Healthcare, Waukesha, WI, USA). Time density curves were obtained and functional maps created, as described previously [8]. Based on a review of existing literature, ischemic core volumes were then calculated using three different thresholds (relative cerebral blood flow [rCBF] <30% from the EXTEND IA, SWIFT PRIME, and DEFUSE-3 trials [3,4,6], cerebral blood flow [CBF] <7 mL/100 g/min based on a meta-analysis by Bandera et al. [10], and cerebral blood volume [CBV] <2 mL/100 g based on a study by Wintermark et al. [9] Penumbra volumes were calculated using the CTP 4D equivalent of RAPID (iSchemaView, RAPID, Menlo Park, CA, USA) Tmax >6 seconds threshold [11].
Follow-up imaging
Depending on the individual center’s follow-up protocol, either diffusion-weighted MRI or NCCT images were obtained 24±6 hours after initial presentation.
Image analysis
Images were assessed visually in three reading sessions (session 1, baseline NCCT only; session 2, baseline NCCT and mCTA; session 3, follow-up MRI/CT) with a 2 weeks interval between each of the sessions. Two raters (J.M.O., O.V.) read the images by consensus. Unclear findings were reviewed and interpreted by a senior neuroradiologist (M.G.). The readers had access to baseline clinical information (clinically affected hemisphere, National Institutes of Health Stroke Scale [NIHSS], time since symptom onset) during all reading sessions. They were blinded to other imaging and clinical outcomes. All CTP studies were interpreted with independent computerized methods. For a more detailed description of the image interpretation methodology see Supplementary material.
Statistical methods
We classified patients by EVT eligibility criteria defined by both: (1) NCCT+mCTA and (2) NCCT, single-phase CTA and CTP. Eligibility was defined by the presence of a large vessel occlusion (LVO) plus adequate collateral scores or appropriate perfusion criteria (Supplementary Table 1). The criteria for mCTA-based eligibility were derived from the ESCAPE and Safety and Efficacy of Nerinetide (NA-1) in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE NA1) trials [2,12]. Three criteria for CTP-based core definition were applied: ischemic core (rCBF <30% [6], CBF <7 mL/100 g/min [10], and CBV <2 mL/100 g [9]). The CTP 4D equivalent of RAPID iSchemaView Tmax >6 seconds was used as penumbra threshold [11]. Cross tables and Venn diagrams were used to show modality-congruence between mCTA and CTP eligibility for EVT. Differences in proportions were assessed using the Fisher’s exact test.
Co-primary outcomes were (1) independent outcome, defined as mRS score of 0–2 at 90 days and (2) major clinical improvement, defined as a 50% relative reduction in the NIHSS score from baseline to 24-hour clinical follow-up. We chose major clinical improvement as a co-primary outcome a priori [8], based on prior published literature [8], since it is more immediate, a direct reflection of treatment success and less likely to be confounded by differences in post-stroke rehabilitation and complications that occur during the 90-day follow-up period.
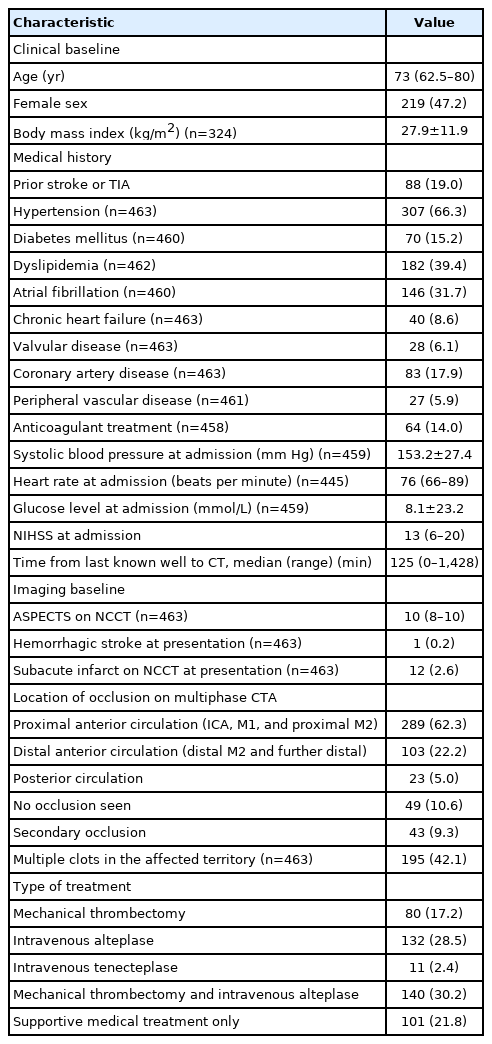
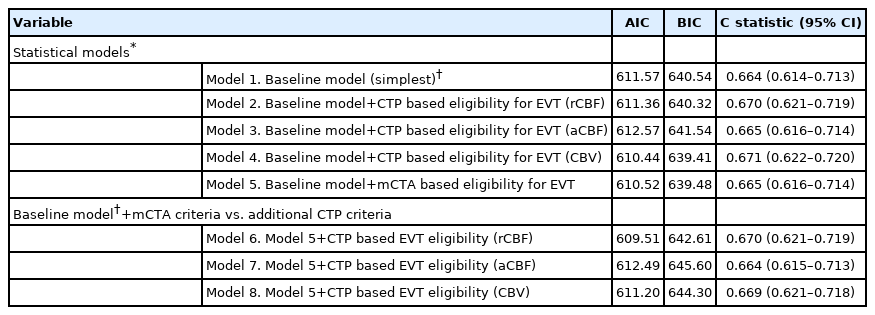
Patient baseline characteristics were reported using descriptive statistics. We constructed and compared a series of logistic regression models to assess the association between each imaging selection paradigm and the two pre-specified outcomes separately. A base model (model 1/“baseline model”) was generated including pre-specified independent variables (age, baseline stroke severity as measured by the NIHSS, time from stroke symptom onset to baseline NCCT, baseline Alberta Stroke Program Early CT Score [ASPECTS; 11 point ordinal scale with lower values indicating greater degree of early ischemic changes] on NCCT, and occlusion location [anterior circulation LVO vs. not] and treatment type [EVT vs. not]). In other words, Model 1 included imaging information from NCCT (ASPECTS) and single-phase CTA only, which is the recommended imaging paradigm for patients with suspected AIS presenting in the early time window as per current guidelines [13,14]. Each subsequent model incorporated imaging-based EVT eligibility criteria defined by each of the imaging threshold approaches described above as binary variables (eligible vs. ineligible). Model 2 included CTP criteria, with an ischemic core threshold of rCBF <30%; Model 3 included CTP criteria, with an ischemic core threshold of rCBF <7 mL/100 g/min; Model 4 included CTP criteria, with an ischemic core threshold of CBV <2 mL/100 g/min; Model 5 included mCTA EVT eligibility criteria. Models 6 through 8 were composite models that included each the above CTP eligibility criteria plus the mCTA EVT eligibility criteria. Akaike information criterion (AIC) and Bayesian information criterion (BIC) were then used to compare information loss across these five statistical models. AIC and BIC estimate the amount of information that is lost by a model while also considering the trade-off between the goodness of fit and simplicity of the model. Lower AIC and BIC scores indicate a better model. The C statistic was determined using receiver operating characteristics (ROC) for each of the above statistical models to describe the discriminative value (patient classification) of each model with higher C-statistics indicating a better model.
To understand the relative predictive value of each imaging criteria further, we constructed an 8-level nominal categorical variable for each of the models above (base model, three CTP approaches, mCTA approach, three CTP plus mCTA approaches). We used logistic regression, adjusted for patient age, baseline NIHSS, time from stroke symptom onset to baseline NCCT, baseline ASPECTS, occlusion location, and a dummy variable representing the eight possible combinations of mCTA eligibility (yes vs. no) versus CTP-eligibility (yes vs. no) conditional on treatment type (EVT performed vs. not) to assess the effect on outcome. This model helps generate probabilities for good outcomes for the following eight conditions namely (1) mCTA eligible, CTP eligible, EVT performed; (2) mCTA not eligible, CTP eligible, EVT performed; (3) mCTA not eligible, CTP eligible, EVT performed; (4) mCTA not eligible, CTP not eligible, EVT performed; (5) mCTA eligible, CTP eligible, EVT not performed; (6) mCTA not eligible, CTP eligible, EVT not performed; (7) mCTA not eligible, CTP eligible, EVT not performed; and (8) mCTA not eligible, CTP not eligible, EVT not performed. We deliberately chose to report adjusted rather than unadjusted outcomes since in a non-randomized setting, the latter are subject to substantial confounding by baseline prognostic variables. The effect size estimate using this approach is then relative to the base model and allows direct comparison of each imaging modality and treatment combination.
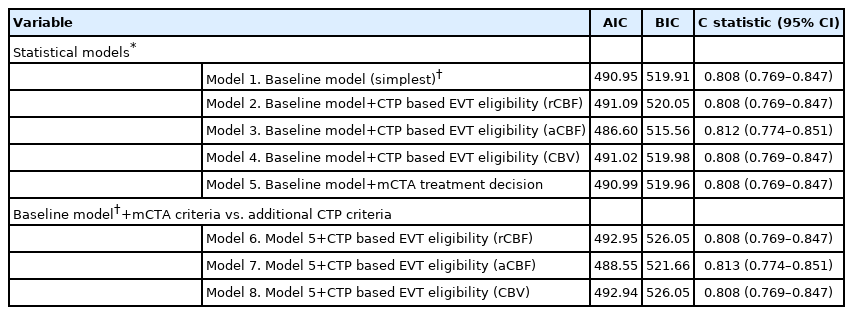
Finally, the net reclassification index (NRI) and integrated discrimination improvement (IDI) were calculated to compare imaging paradigms in predicting outcome to provide an estimate of the relative incremental benefit of each imaging approach. All analyses were performed in Stata version 15.1 (StataCorp., College Station, TX, USA). Two-sided P-values <0.05 were considered statistically significant. Results were visualized using Microsoft Excel version 16.18 (Microsoft, Redmond, WA, USA).
Results
Study participants and outcomes
Of the 595 patients enrolled in the study, complete clinical and imaging information were available in 464 patients, who were included in this analysis (Supplementary Figure 1). Patient baseline characteristics and treatment details are shown in Table 1. A detailed overview of the occlusion sites on baseline CTA is provided in Supplementary Table 2. Overall, 220 (47.4%) patients were treated with EVT, either as single treatment or in combination with intravenous alteplase. Clinical outcome data are shown in Supplementary Table 3.
Comparison of eligibility for EVT based on mCTA vs. CTP criteria
Venn diagrams illustrating mCTA and CTP eligibility proportions when compared to the sample with LVOs and the entire sample are shown in Figure 1. Of 289/464 patients with LVO on CTA, 263 (91%) were eligible for EVT by mCTA eligibility criteria versus 63 (22%), 19 (7%), and 103 (36%) by rCBF, absolute cerebral blood flow (aCBF) and CBV CTP criteria. CTP and mCTA criteria were discordant in 44% (204/464, rCBF <30%) (Figure 1A), 53.4% (248/464, aCBF <7 mL/100 g/min) (Figure 1B), and 39.7% (184/464, CBV <2 mL/100 g/min) (Figure 1C), respectively. Most discordant patients were classified as EVT-eligible based on mCTA but not on CTP, regardless of the CTP threshold applied (rCBF, 99.0% [202/204]; aCBF, 99.2% [246/248]; CBV, 93.5% [172/184]) (Figure 1). Results were very similar when only patients presenting beyond 6 hours (n=69) were included (Supplementary Figure 2).

Proportional Venn diagrams illustrating the number of patients with anterior circulation large vessel occlusion (LVO), i.e., target for endovascular treatment (EVT; grey circles), and EVT eligible patients based on computed tomography perfusion (CTP) criteria (blue circles) and multi-phase computed tomography angiography (mCTA) criteria (green circles). (A) Illustrates CTP based EVT-eligibility for relative cerebral blood flow (rCBF) <30% as core threshold, (B) illustrates CTP based EVT-eligibility for absolute cerebral blood flow (aCBF) <7 mL/100 g/min as core threshold, and (C) illustrates CTP based EVT-eligibility for absolute cerebral blood volume (aCBV) <2 mL/100 g as core threshold.
Association between imaging selection paradigms for EVT and clinical outcomes
Modelling results with the two co-primary clinical outcomes as dependent variables are shown in Tables 2 and 3. When assessing 90-day mRS 0–2 as outcome, information loss was lowest with the CTP (aCBF) model (AIC, 486.60; BIC, 515.56) with the mCTA model being next lowest (AIC, 491.00; BIC, 519.96). Information loss with the other CTP based models was mostly higher and the C statistic lower than the rCBF and the mCTA based models (although the 95% confidence intervals [CIs] for the C statistics overlap). When assessing major clinical improvement at 24 hours as outcome, information loss was lowest with the CTP (CBV) model (AIC, 610.44; BIC, 639.41) with the mCTA model being next lowest (AIC, 610.52; BIC, 639.48) while the 95% CI of the C statistics for all models overlapped. Results were similar after excluding posterior circulation strokes (data not shown) and in subjects with only anterior circulation LVO (Supplementary Table 4).
Estimated probabilities of pre-defined clinical outcomes (along with interquartile range), adjusted for pre-specified baseline variables and CTP versus mCTA eligibility criteria conditional on receiving EVT treatment, are shown in Figure 2. On average, in patients who received EVT, estimated outcomes were best in patients who met both mCTA and CTP eligibility criteria for EVT (62% to 87% good outcome rate and 57% to 63% rate of major clinical improvement depending on the CTP threshold used). Patients eligible for EVT by mCTA criteria and not by CTP criteria who received EVT achieved adjusted good outcome rates of 53% to 57% and major clinical improvement rates of 55% to 58%. Very few patients met CTP eligibility criteria and not mCTA eligibility criteria for EVT (n=2, 2 and 12 for the rCBF, aCBF, and CBV based thresholds, respectively). Patients who did not meet either mCTA or CTP eligibility criteria and received EVT achieved adjusted good outcome rates of 51% to 62% and major clinical improvement rates of 42% to 49%. Patients who met CTP or mCTA eligibility criteria but were not offered EVT were mainly patients with mild symptoms (NIHSS ≤7), pre-stroke disability (baseline mRS ≥2), long onset to imaging times (>6 hours), extensive ischemic changes on baseline imaging (ASPECTS 6 or 7) and patients above 80 years of age (data not shown). Almost all patients (n=150/156, 96%) who did not meet mCTA or CTP eligibility criteria and were not offered EVT did not have a LVO in the anterior circulation.
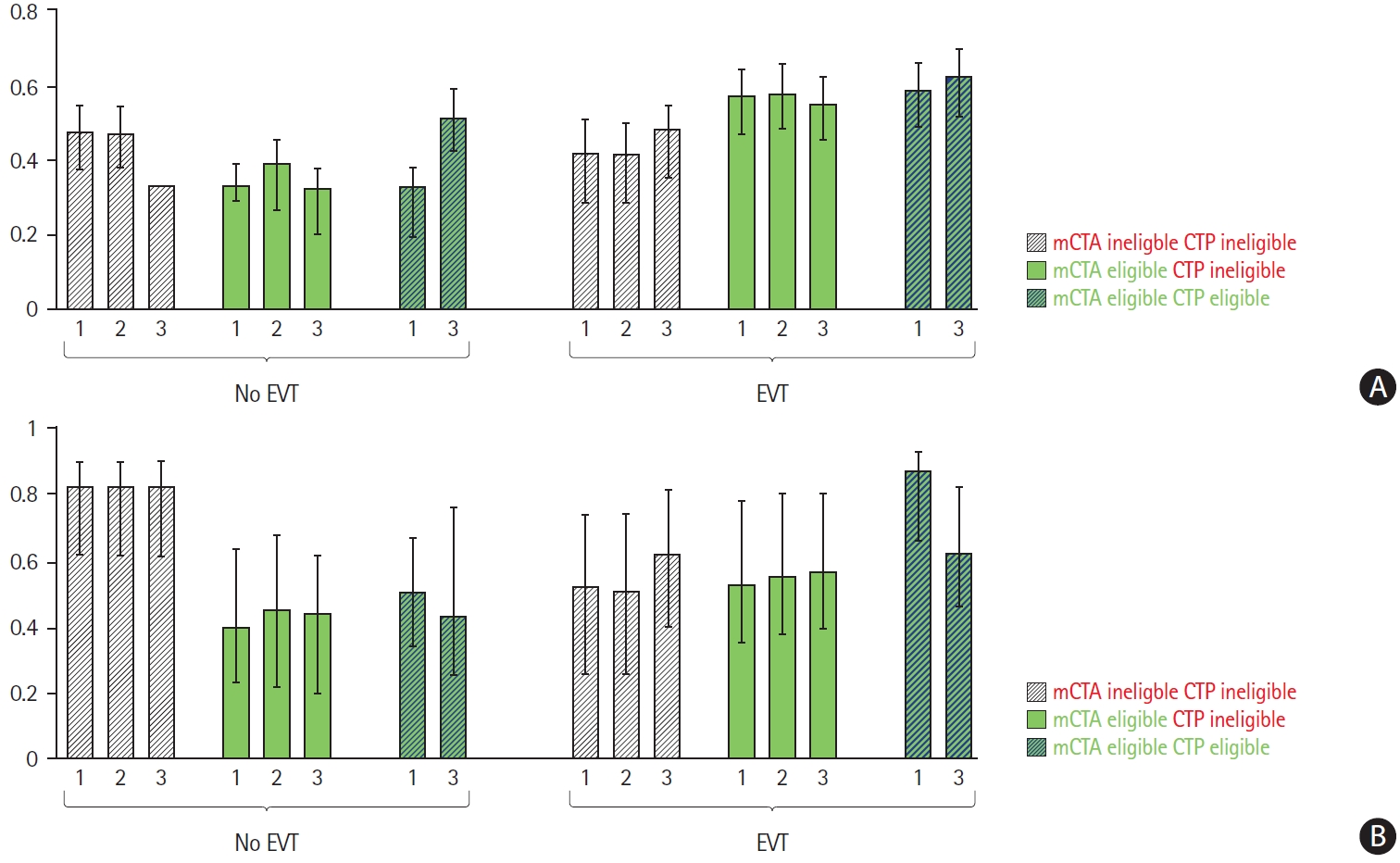
Median adjusted probability (model estimated) of major clinical improvement (A) and good outcome (B) conditional on computed tomography perfusion (CTP) vs. multi-phase computed tomography angiography (mCTA) based imaging selection criteria vs. both vs. neither and by treatment offered (endovascular treatment [EVT] vs. no EVT). Bars labelled with indicate probabilities for relative cerebral blood flow <30% as core threshold; bars labelled with indicate probabilities for absolute cerebral blood flow <7 mL/100 g/min as core threshold; and bars labelled with indicate probabilities for cerebral blood volume <2 mL/100 g as core threshold. Note that groups with less than 20 patients are not represented in this figure.
Net reclassification analysis
When comparing a statistical model that included baseline clinical information, NCCT ASPECTS, LVO presence, and mCTA based EVT eligibility criteria to another model that included the CTP based EVT eligibility criteria in addition, the NRI and IDI for major clinical improvement were small and not statistically significant (Table 4). The NRI was negative when rCBF <30% and CBV <2 mL/100 g were used as ischemic core threshold, implying a worsening rather than an improvement in reclassification. Results for mRS 0–2 at 90 days were similar, except when aCBF <7 mL/100 g/min was used; here, the IDI was small but significant.
Discussion
This study finds that nine of every 10 patients with LVO presenting within 12 hours of symptom onset are considered eligible for EVT by mCTA criteria when compared to two of every 10 subjects considered eligible for EVT by rCBF based CTP criteria, four of every 10 subjects by CBV CTP criteria and one of every 20 subjects by aCBF CTP criteria. Clinical outcomes are likely best when patients meet both mCTA and CTP eligibility criteria for EVT and are offered EVT (57% to 63% rate of major clinical improvement, 62% to 87% rate of good outcome, depending on the CTP threshold used). Clinical outcomes in patients who meet mCTA eligibility criteria for EVT but not CTP eligibility criteria (55% to 58% rate of major clinical improvement, 53% to 57% rate of good outcome) and in patients who do not meet either mCTA or CTP eligibility criteria (major clinical improvement rates of 42% to 49% and good outcome rates of 51% to 62%) are also good if they are offered EVT.
Acute stroke imaging serves two major purposes: treatment decision-making and prognostication. As opposed to prognostication, treatment decision making (patient selection for therapy) is a dichotomous process; either a decision is made in favor of, or against treatment. If imaging selection is to be considered valid, patients who are considered ineligible for treatment based on imaging should not benefit from that treatment. With the arguable exception of the MR CLEAN trial, all randomized controlled trials (including the late window DAWN and DEFUSE-3 trials) showing benefit of EVT in patients with LVOs used imaging selection criteria to exclude patients with LVOs from EVT. These imaging selection criteria range from the stringent e.g. CTP core and mismatch based criteria to the less stringent, e.g., mCTA or single phase CTA based criteria with NCCT ASPECTS criteria. Current guidelines suggest that additional imaging beyond NCCT and single-phase CTA is not required in the early time window [13,14]. However, in current practice, CTP is often routinely obtained in all patients with suspected AIS, and there are several studies investigating the role of perfusion imaging in the early time window [15,16], including the ongoing randomized A Randomized Controlled Trial to Optimize Patient's Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT-2) trial (NCT03876457) [17]. Our results show the potential impact of some of these various imaging selection criteria in excluding patients with LVOs presenting within 12 hours of last known well from EVT. More stringent imaging selection criteria result in better outcomes on average in patients selected for and offered EVT but at the cost of significantly less patients being offered EVT at the population level. Progressively less stringent imaging selection criteria result in more patients being offered EVT at the population level. Of note, even with the simplest imaging selection criteria that just seek to identify the existence of target LVO in patients, one in two patients achieve good clinical outcomes when offered EVT. In an earlier study, Kim et al. [18] compared mCTA and CTP based triage in late window patients with confirmed LVO using the RAPID software, which defines ischemic core using an rCBF threshold of <30% and a penumbra threshold of Tmax threshold of >6 seconds, and defined CTP based EVT eligibility using the DAWN and DEFUSE-3 criteria. They concluded that mCTA based EVT triage may be a good alternative to CTP based patient selection in the late time window. The present study evaluated additional CTP criteria and included a broader patient sample (patients with LVO, but also those with medium and small vessel occlusions, lacunar strokes, and stroke mimics), presenting both in the early and late time window. This study therefore provides additional, confirmatory evidence that imaging strategies like NCCT/CTA or mCTA may be adequate for imaging based EVT triage, both in early and late time windows.
The fact that our results show approximately 50% to 62% good clinical outcome and 42% to 49% major clinical improvement rates in patients who are deemed ineligible for EVT by CTP or mCTA imaging criteria but were treated with EVT (when compared to 19% good clinical outcome in the control arm of the MR CLEAN trial [1] and 26.5% good clinical outcome in the control arm of the HERMES meta-analysis [19]) attests to the need for more careful consideration of the impact that current imaging selection criteria may have on health outcomes at a population level by excluding patients from robust treatments such as EVT. Other more recent analyses also support the idea that use of less stringent imaging selection criteria is likely to result in more patients receiving EVT and potentially benefiting from it, even in patients who present late [20]. This raises the question as to what is then the use of more advanced imaging in acute stroke decision making? Our results show that, irrespective of the time window when patients present, statistical models for outcome determination that include imaging information from mCTA, CTP or a combination of these modalities have lower information loss and potentially better discrimination than models that do not include such information and rely only on ASPECTS and the presence of an LVO (the recommended triage strategy for early time window patients as per current guidelines), although the differences between these various models are minor. This suggests that information from more advanced imaging, i.e., imaging information that goes beyond mere detection of vessel occlusions, seems to have some additional utility for prognostication in patients with AIS.
Our results also show that very few patients (≤3%) were eligible for EVT by CTP but not by mCTA. A patient level pooled analysis of data from the HERMES consortium of all recent EVT trials showed that patients with large ischemic cores on CTP may also benefit from EVT [21]. A revision of current CTP criteria with a larger volume threshold for ischemic core and more liberal mismatch criteria may therefore agree more with the mCTA eligibility criteria presented here. Of note however, analysis of imaging criteria such as ASPECTS on NCCT and collateral status on CTA from the HERMES consortium did not show any treatment effect modification by imaging criteria, thus suggesting that patients who would otherwise be deemed ineligible for EVT by these imaging criteria may still benefit from EVT [22]. Ongoing randomized controlled trials such as Efficacy and safety of ThrombEctomy iN Stroke with extended leSION and extended time window (TENSION), IN EXTREMIS, and Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke (TESLA) are likely to shed further light on the appropriate use of imaging for patient selection for EVT.
A major strength of this study is that results are derived from a prospective multi-center cohort study with a large sample size and broad inclusion criteria. There are however some limitations to this study. First, patients were not randomly allocated to EVT versus medical management. We therefore do not know the “alternative” outcome had they been treated differently and could not directly compare the impact of CTP versus mCTA based EVT decision making on clinical outcome. Doing so would warrant a large diagnostic randomized controlled trial [23]. Such a trial would be a pre-requisite for establishing the best imaging selection strategy for patients with LVOs presenting in the late time-window, but it would also be challenging to conduct, due the large required sample sizes and other methodological considerations [23]. Second, assessment of CTP based EVT criteria in our study was fully automated but mCTA based EVT eligibility relied on visual scoring of collateral status. This may raise concern about between reader variability in assessing collateral status on mCTA. Although previous studies have shown that collateral assessment on mCTA by human raters may have good inter-rater reliability [8,18], automated collateral scoring systems that are increasingly available, will likely further mitigate concerns about reliability of such collateral assessment [24]. Third, we used three different ischemic core definitions to represent a variety of currently used CTP parameters. Although these definitions are the most commonly used, the use of other ischemic core and penumbra thresholds may lead to different results. Fourth, only 69 subjects in this study presented beyond 6 hours of stroke symptom onset. Although we have shown previously that in these patients, the use of mCTA compares well with CTP in selecting patients for EVT [25], more data will be needed to compare these various available imaging paradigms in the late time window. The multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in The Netherlands for late arrivals (MR CLEAN LATE) (ISRCTN19922220) randomized controlled trial will provide further evidence in this regard.
Conclusions
In conclusion, this study suggests that simpler imaging selection criteria that rely on little else than detection of the occluded blood vessel may be more sensitive and less specific at a population level, thus resulting in more patients being offered EVT and arguably benefiting from it in patients with AIS and LVOs presenting within 12 hours of last known well. Advanced imaging however may have some additional value for prognostication in these patients.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2021.00619.
EVT-eligibility criteria for different imaging modalities
Detailed location of intracranial vessel occlusion sites (n=464)
Clinical and imaging outcomes of the study sample (n=464)
Predicting major clinical improvement with imaging
Flow chart of initially enrolled and excluded patients. mCTA, multi-phase computed tomography angiography; CTP, computed tomography perfusion.
Proportional Venn diagrams illustrating the number of patients with anterior circulation large vessel occlusion (LVO), i.e., target for endovascular treatment (EVT; grey circles), and EVT eligible patients based on computed tomography perfusion (CTP) criteria (blue circles) and multiphase computed tomography angiography (mCTA) criteria (green circles) in patients presenting beyond 6 hours (n=69). (A) illustrates CTP based EVT-eligibility for relative cerebral blood flow (rCBF) <30% as core threshold, (B) illustrates CTP based EVT-eligibility for absolute cerebral blood flow (aCBF) <7 mL/100 g/min as core threshold, and (C) illustrates CTP based EVT-eligibility for absolute cerebral blood volume (aCBV) <2 mL/100 g as core threshold. Note that the slight mismatch between the right margin of the green and grey circles in (C) is related to the diagram type; there were no patients without LVO who are EVT eligible based on mCTA criteria.
Notes
Disclosure
Mayank Goyal: consultant (Medtronic, Stryker, Microvention, GE Healthcare, Mentice). Michael Hill: grants from Stryker, Medtronic, NoNO Inc., Boehringer Ingelheim. Bijoy Menon: patent (system/method for decision making/triaging in acute stroke). Johanna Ospel: research scholarships from the University of Basel Research Foundation, Julia Bangerter Rhyner Foundation and “Freiwillige Akademische Gesellschaft Basel.” The remaining authors have nothing to disclose.
Acknowledgements
This study was supported by a grant from the Canadian Institute of Health Research. The authors are most grateful to all enrolling sites.