Cerebral Microbleeds in Autosomal Dominant Polycystic Kidney Disease
Article information
Dear Sir:
Autosomal dominant polycystic kidney disease (PKD) is characterized by multiple enlarged renal cysts that subsequently lead to renal insufficiency [1]. While PKD is associated with intracranial aneurysm [2], which reflects large artery anomalies, it remains unknown whether there is also abnormality in cerebral small vessels. The aim of this study was to investigate the prevalence and determinants of cerebral microbleeds (CMBs) in a cohort of patients with PKD.
Fifty-five consecutive PKD patients without past history of stroke, and 45 control subjects who visited the neurology outpatient clinics with no prior stroke or family history of PKD, were prospectively enrolled for brain magnetic resonance imaging (MRI), including susceptibility-weighted imaging (SWI). Twenty-two PKD patients (40%) received another follow-up brain MRI study. CMBs were defined as small, round or ovoid hypointensities with a diameter less than 1 cm on SWI [3]. A subgroup of patients with PKD underwent genetic testing using next-generation sequencing to screen the entire PKD1 and PKD2 genes. Fisher’s exact test and Student's t-test or Mann-Whitney U test were used to determine the differences between groups for categorical data and continuous data, respectively. The determinants for the presence of CMBs were analyzed using a logistic regression model. Two-tailed P-values of less than 0.05 were considered statistically significant (STATA software version 8.2, StataCorp., College Station, TX, USA). Detailed methods are available in Supplementary methods [1,3-12].
Comparison of the 55 PKD patients with the 45 control subjects (Supplementary Table 1) showed that PKD patients had a lower estimated glomerular filtration rate (eGFR) level than control subjects. More patients in the PKD group had CMB (27.3% vs. 6.7%, P=0.009), intracranial aneurysm (10.9% vs. 0%, P=0.031) and hypertension than in the control group. Among 15 PKD patients with CMBs (mean age, 58.4±10.8 years; range, 30 to 73), seven patients (46.7%) had CMBs located exclusively at the lobar regions, five (33.3%) had CMBs located exclusively at the deep regions and three (20%) had lobar and deep CMBs (mixed location). The number of CMBs for each patient ranged from 1 to 6, with a total count of 40 CMBs for all patients; 22 (55%) of the CMBs were in the lobar regions (12 in the frontal, five in temporal, four in occipital, and one in parietal regions) and 18 (45%) were in the deep regions (eight in the cerebellum, five in basal ganglion, three in brainstem, and two in thalamus) (Figure 1).
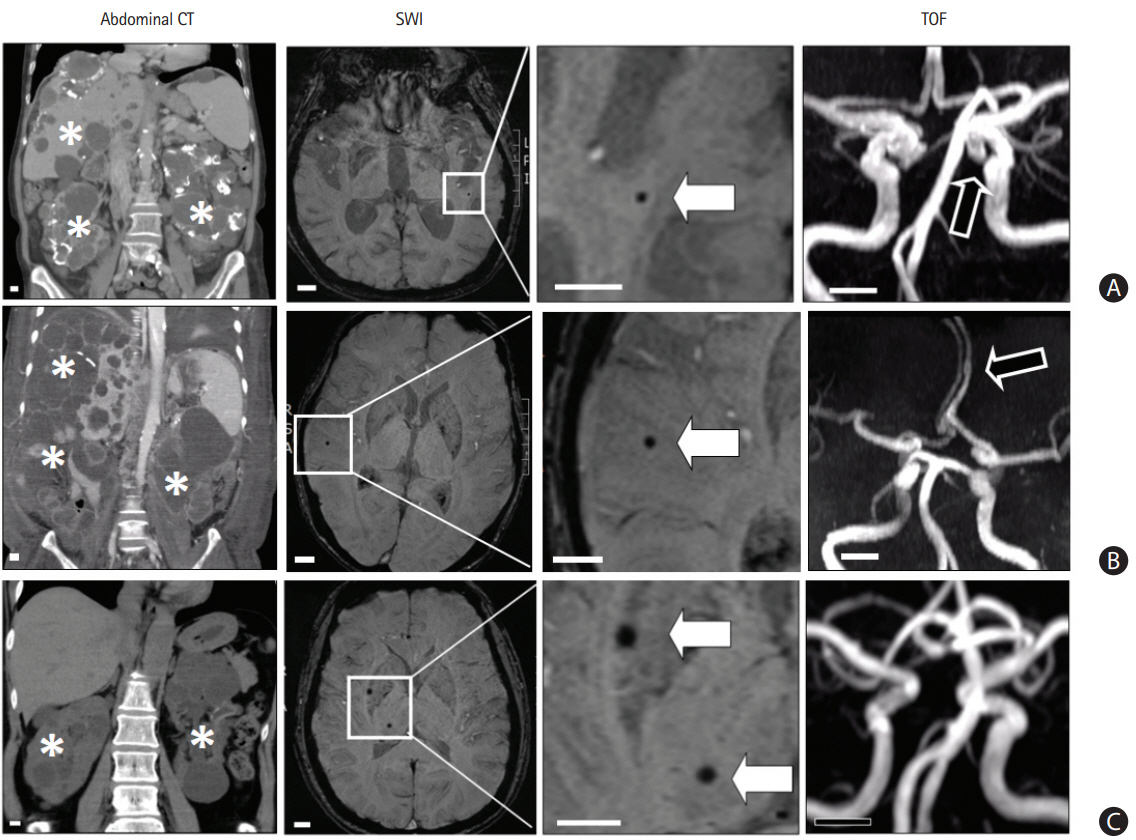
Patients with polycystic kidney disease (PKD) and having cerebral microbleeds (CMBs). (A) A 70-year-old woman had PKD with multiple cysts in bilateral kidney and liver (asterisks) noted by abdominal computed tomography (CT). The susceptibility-weighted imaging (SWI) shows a CMB (white arrow) in the left temporal area. The time-of-flight (TOF) image shows an intracranial aneurysm (black arrow) in the left distal internal carotid artery. (B) A 50-year-old man had PKD and the SWI shows a CMB (white arrow) in the right temporal area. The TOF image shows a fusiform aneurysm (black arrow) in the left anterior cerebral artery. (C) A 55-year-old woman had PKD and the SWI shows two CMBs (white arrows) in the right basal ganglion and thalamus. The TOF image does not show an aneurysm. Scale bar, 1 cm.
Comparing the characteristics of PKD patients with and without CMBs (Supplementary Table 2), PKD patients with CMBs were older than those without (58.4±10.8 years vs. 48.7±9.8 years, P=0.002). In univariable analysis of 100 patients and controls, age and the diagnosis of PKD were the positive determinants and eGFR level was a negative determinant for the detection of CMBs (Table 1). Using a multivariable logistic regression model, only older age (adjusted odds ratio [aOR], 1.09; 95% confidence interval [CI], 1.02 to 1.17) and the diagnosis of PKD (aOR, 7.28; 95% CI, 1.18 to 45.0) remained independent determinants for the presence of CMBs.
In 49 patients receiving genetic testing, 22 (44.9%) and 18 patients (36.7%) were found to have PKD1 and PKD2 gene causative variants, respectively (Figure 2). No significant genotype correlated with CMBs in PKD patients. In 22 PKD patients who received the second MRI study with an average follow-up period of 36.5±12.4 months, seven (31.8%) developed new CMBs. For 12 PKD patients with no CMBs noted on the first MRI study, only one (8.3%) had new CMBs on the follow-up MRI study; however, six of 10 PKD patients (60%) with CMBs on the initial MRI developed new CMBs (P=0.02). Patients with new CMBs during follow-up were older than those without new CMBs (60.9±8.4 years vs. 50.2±8.9 years, P=0.024).

Genotype-phenotype correlation in 49 patients with polycystic kidney disease (PKD). (A) Twenty-two (44.9%) and 18 (36.7%) patients were found to have PKD1 and PKD2 gene causative variants, respectively. (B, C) The risk of having cerebral microbleeds (CMBs) did not differ significantly by the presence of different causative genes (P=0.84) or variant types (P=0.5). (D) For PKD1 causative variants, no specific gene region (5’:1–6,000 vs. 3’:6,000–12,000) was associated with CMBs (P=0.62). (E) Among PKD patients who had CMBs and received a genetic study (n=13), patients with the PKD1 causative variant showed a trend to have a higher average number of CMBs than those with PKD2 causative variants (3.8±2.0 vs. 1.4±0.5, P=0.08). The total number of PKD patients with CMBs was relatively small (n=15), impeding a complete phenotype and genotype analysis.
The prevalence of CMBs in our PKD patients was about one-fourth (27.3%), much higher than that in the normal population of this study (6.7%). Multivariable analysis revealed that older age and PKD diagnosis were the independent determinants for the presence of CMBs. During long-term follow-up, about one-third (31.8%) of PKD patients also developed new CMBs. The present study demonstrates a novel vascular phenotype of PKD, i.e., CMBs, which indicates the relative structural fragility of cerebral small vessels in those with PKD.
Patients with cerebral small vessel disease (SVD) have been reported to more likely have CMBs. The present study is the first to demonstrate that those with PKD are prone to have CMBs. Other MRI markers found commonly in SVD, including an increase of white matter hyperintensity, the presence of lacune, and a high degree of enlarged perivascular space [3], were not features in PKD patients. The cerebral small vessel fragility in PKD might be the underlying pathophysiology that results in only CMB without any other parenchymal injury.
The distribution of CMBs in PKD patients were heterogeneous and found exclusively at lobar locations, exclusively deep and mixed regions in 46.7%, 33% and 20% of patients, respectively. In seven patients with exclusively lobar CMBs, three were younger than 55 years old and did not fulfill the Boston diagnostic criteria for cerebral amyloid angiopathy (CAA) [13]. Therefore, CAA is less likely in these relatively young patients when coexisting CAA is possible. Although chronic hypertension may be a risk factor for CMB formation in PKD patients, the fact that more than half of the CMBs were located in the lobar area suggests that factors other than hypertension may cause CMB formation. In addition, neither the presence of hypertension nor the eGFR level was significantly associated with CMBs in the multivariable model.
In summary, we report a novel phenotype of CMBs in patients with PKD that indicates the fragility of cerebral small vessels involving both deep penetrating and cortical-subcortical regions. The prevalence of CMBs is about one-fourth of PKD patients. Older age and the diagnosis of PKD are the only independent determinants of the occurrence of CMBs. Brain MRI studies in patients with PKD should emphasize the presence of not only intracranial aneurysm, but also CMBs.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2019.02838.
Supplementary methods
Characteristics of patients with and without polycystic kidney disease
Characteristics of patients having polycystic kidney disease with and without cerebral microbleeds
