 |
 |
- Search
| J Stroke > Volume 24(3); 2022 > Article |
|
Abstract
Transcatheter patent foramen ovale (PFO) closure is a safe and effective treatment for secondary prevention after a PFO-associated stroke as demonstrated in multiple large randomized clinical trials. However, these trials excluded a significant proportion of patients who could have benefited from percutaneous PFO closure due to coexisting potential confounders such as additional thromboembolic risk factors, namely thrombophilia. Since scarce and conflicting data existed on such patients, current clinical management guidelines on patients with PFO mainly recommended against PFO closure in patients with thrombophilia and failed to provide any recommendation on the type and duration of antithrombotic treatment after transcatheter PFO closure. In the past 2 years, there has been new evidence supporting transcatheter PFO closure as a clinically meaningful alternative (vs. medical treatment) in this high-risk group of patients, along with additional data supporting the important role of systematic screening for thrombophilia in PFO-associated cerebrovascular events. This review article provides an updated overview of the incidence, clinical characteristics and outcomes of PFO closure in patients with thrombophilia, also commenting on the most appropriate medical treatment after PFO closure and future perspectives in the field.
Transcatheter patent foramen ovale (PFO) closure is a safe and effective treatment for secondary prevention after a PFO-associated stroke [1]. This has been extensively demonstrated in large randomized clinical trials which excluded a significant proportion of patients who could have benefited from percutaneous PFO closure due to coexisting potential confounders such as additional thromboembolic risk factors, namely thrombophilia. The prevalence of thrombophilia in the general population ranges between 0.1% and 20% [2,3], and the prevalence of PFO from 20% to 34% [4]. Interestingly, the prevalence of both conditions is higher in stroke patients compared to the general population, and both have been associated with a higher risk of cerebrovascular events [5]. Due to scarce and inconsistent clinical evidence [6], and to the exclusion of patients with thrombophilia from most PFO closure randomized clinical trials, current guidelines regarding the management of PFO-stroke patients failed to make strong recommendations on transcatheter closure in such patients along with the lack of indication for thrombophilia screening in this setting [7,8].
In the past 2 years, there has been new evidence supporting transcatheter PFO closure as a clinically meaningful alternative (vs. medical treatment) in this high-risk group of patients [9-11], along with additional data supporting the important role of systematic screening for thrombophilia in PFO-associated cerebrovascular events. This review article provides an updated overview of the incidence, clinical characteristics and outcomes of PFO closure in patients with thrombophilia, also commenting on the most appropriate antithrombotic treatment after PFO closure and future perspectives in the field.
Thrombophilia is defined as an abnormality of the coagulation or fibrinolytic system that results in a hypercoagulable state (HCS) increasing the risk of venous or arterial intravascular thrombus and thromboembolic events [2,3,12,13].
The predisposition for intravascular thrombus may arise from heritable genetic mutations (inherited thrombophilias) or acquired factors (acquired thrombophilias, like trauma, surgery, malignancy, autoimmune diseases, etc.), which would lead to a hypercoagulability state due to an excess or hyperfunction of a procoagulant factor or a deficiency of an anticoagulant moiety [3]. Inherited thrombophilia carries a life-long higher risk of thromboembolic events. On the other hand, some acquired thrombophilias are transient and can be treated (e.g., hyperhomocysteinemia without mutation of methylenetetrahydrofolate reductase [MTHFR] gene when treating vitamin B deficiencies) but others continue to carry a life-long risk of thromboembolic events (e.g., antiphospholipid syndrome) [3]. Some types of thrombophilias have been shown to cause venous thromboembolic events only (e.g., factor V Leiden, protein C deficiency, etc.) whereas others can cause mixed (arterial, venous) thromboembolic events, (e.g., antiphospholipid syndrome or mutation of the MTHFR gene) [3].
The prevalence of a specific type of thrombophilia in the general population varies greatly in the literature, ranging from 0.1% to 20% depending on different factors, such as definition, tests used for diagnosis, use of confirmatory tests, etc [2,3]. The reported increased risk of thromboembolic events in thrombophilia patients has been highly variable, ranging from 0.3- to 100-fold, varying according to several factors such as the type of thrombophilia, carrier status (homozygous vs. heterozygous), reversibility, and the number of thrombophilia disorders [2]. The classification, prevalence, and estimated risk, alongside their inherited or acquired forms for the types of thrombophilias related to a PFO-associated stroke and their supporting evidence are shown in Table 1 [2-4,10,14-28].
The presence of a PFO has been shown to be a common finding in the general population, with a prevalence between 20% and 34% [4]. PFO is detected more frequently in young adults with an ischemic stroke of undetermined etiology (54% to 56%) compared to non-stroke controls (10% to 18%) [14]. A meta-analysis including 23 case-control studies in patients younger than 55 years showed a strong association between the presence of a PFO and cryptogenic stroke, the nowadays referred as “PFO-associated stroke,” [15] compared to stroke of known etiology (odds ratio [OR], 5.1; 95% confidence interval [CI], 3.3 to 7.8) [16].
Two mechanisms have been proposed to explain the role PFO in systemic embolism: (1) paradoxical embolism, which implies the passage of a right-sided venous thrombus to the left circulation through the PFO, specially during transient or persistent elevations on right-heart pressures which would allow to the passage of blood (or thrombus) from the right atrium to the left atrium, and (2) in situ PFO thrombus formation, which can lead to embolization to the left circulation [4,29]. Both mechanisms can be enhanced in the setting of thrombophilia, which may increase by several times the risk of thrombus formation in the venous system or in the PFO. Both circumstances have been studied and further characterized, leading to the identification of high-risk features for PFO including a large PFO (maximum separation of the septum primum from the secundum >2 mm), long tunnel (>10 mm), atrial septal aneurysm (hypermobility of the septum with >10 mm excursion), strongly positive bubble study, and prominent Eustachian valve [4]. The co-existence of a prothrombotic steady state such as thrombophilia with a PFO, particularly in the presence of high-risk anatomical features, would potentially increase the risk of systemic thromboembolic events.
Patients with inherited or acquired thrombophilia represent a unique challenge in the setting of stroke and PFO. The combination of a hypercoagulability state and a PFO is not uncommon in clinical practice, with studies on patients with PFO showing a thrombophilia prevalence ranging from 5% to 31% [17-19,30]. This large variability highlights the importance of systematic screening for thrombophilia in patients with PFO since the proper use of screening in this high-risk population can lead to the accurate identification of patients with an even higher risk for a systemic thromboembolic event. In a meta-analysis of thrombophilia in the setting of PFO showed an increased risk of stroke in patients with PTG20210A mutation and PFO compared to controls (OR, 3.85; 95% CI, 2.22 to 6.66) and to patients with cryptogenic stroke without PFO (OR, 2.31; 95% CI, 1.20 to 4.43) [20]. This was recently shown in a survival analysis of patients with PFO-related cerebrovascular events where a higher incidence of recurrent events (composite of stroke or transient ischemic attack [TIA]) was observed in patients with thrombophilia (15.7% vs. 8.3%; hazard ratio [HR], 1.85; 95% CI, 1.09 to 3.16; P=0.024) [9]. In thrombophilia with a predominant increased risk of arterial thrombosis, the effect of PFO closure on recurrent events would be uncertain since closing the shunt may not be associated with any significant impact on paradoxical embolism. However, the in situ thrombus formation (at the level of the PFO) may remain a possible cause.
The first report on transcatheter PFO closure in patients with thrombophilia was reported in 2004 by Giardini et al. [6]. The study included 72 consecutive patients, and 20 patients (28%) were diagnosed with at least one hypercoagulability finding after a systematic screening for thrombophilia. As shown in Table 2 [6,9,10,31], PFO closure has been associated with a 99% success rate along with a very low rate of periprocedural complications, and a very low incidence of recurrent events at 2-year follow-up, with no differences between patients with and without thrombophilia. Interestingly, patients with thrombophilia had also a higher rate of recurrent events before PFO closure compared to those without thrombophilia (P<0.0001), despite a similar follow-up and regardless of shunt severity, presence of an atrial septal aneurysm, and cardiovascular risk factors (Figure 1).
The most recent clinical management guidelines in patients with PFO from the European Society of Cardiology (ESC) [7] and the American Academy of Neurology (AAN) [8] barely addressed the management of patients with thrombophilia, and guideline recommendations stated that the role of thrombophilia on a PFO-related clinical event cannot be generalized and that transcatheter PFO closure and routine thrombophilia screening methods are not generally warranted due to the “previous conflicting” clinical evidence. This has led to a large lack of screening for thrombophilia in stroke-PFO patients, recently reported by a multinational survey showing that 68% of cardiologists failed to perform a systematic screening for thrombophilia in this setting [21]. Current guideline recommendations are summarized in Table 3.
The ESC position statement recommendations on routine laboratory tests for prothrombotic states (thrombophilia testing) stated that these tests are not generally warranted to guide the need for permanent oral anticoagulation (OAC) [7], whereas the AAN statement is that in patients being considered for PFO closure, clinicians should perform hypercoagulable studies that would be considered a plausible high-risk stroke mechanism leading to a change in management such as requiring lifelong anticoagulation [8].
According to ESC guidelines, in the setting of hypercoagulability, deep vein thrombosis and/or pulmonary embolism, PFO closure may be considered when there is a need for temporary OAC or a high risk of recurrence despite permanent OAC, particularly in pulmonary embolism cases, where PFO was reported to be an independent predictor of new brain lesions at follow-up despite optimal OAC [7]. The AAN statement on patients who would otherwise be considered good candidates for PFO closure but require long-term anticoagulation because of suspected or proven hypercoagulability (defined as thrombophilia, unprovoked deep venous thrombosis, or unprovoked pulmonary embolism), is that clinicians should counsel the patient that the efficacy of PFO closure in addition to anticoagulation cannot be confirmed or refuted [8].
Since the publications of the last ESC and AAN guidelines, there has been new evidence of high clinical relevance regarding thrombophilia screening and most importantly, to the indication of transcatheter PFO closure in this high-risk population, including new evidence suggesting a role of PFO closure in primary prevention. This new evidence may help to clarify some controversial results from historical data and would support a new approach for the management of these patients.
The screening for thrombophilia in the overall cryptogenic stroke population, including a small proportion of patients with PFO, has been recently evaluated by Omran et al. [22]. These authors evaluated the ability of genetic and serological testing to diagnose clinically relevant thrombophilia in young adults with ischemic stroke. They performed a retrospective study on young patients diagnosed with acute ischemic stroke at a comprehensive stroke center with laboratory testing for thrombophilia. The primary outcome was a positive thrombophilia screening test. The secondary outcome was a change in clinical management based on thrombophilia testing results using logistic regression to assess prespecified risk factors (age, sex, prior venous thromboembolism, family history of stroke, stroke subtype, and presence of PFO) for changes in prescription of anticoagulation or PFO closure. Among 196 young ischemic stroke patients, including 40 patients with PFO, at least one positive thrombophilia test was identified in 85 patients (43%), 17 (28%) of which had also a PFO. Of those patients with a positive thrombophilia test, 16 (8%) had a resultant change in the management including the initiation of anticoagulation in 10 (63%) and PFO closure in six (37%). The most common thrombophilia screening test leading to a change in the treatment was the detection of antiphospholipid antibodies, which were present in 20 patients (10% of the total cohort).
Lim et al. [14] performed a prospective study on patients who had a stroke/TIA in the setting of PFO and/or interatrial septal aneurysm (IASA) and excluded all other potential causes/conditions related to the cerebrovascular event. A comprehensive arterial and venous thrombophilia screening was performed to determine the risk of recurrent TIA, stroke or cardiovascular death in patients with a PFO using a modern ‘goal-directed’ secondary prevention treatment, including active management of other vascular risk factors, with or without PFO closure. A total of 83 patients were included with a median follow-up of about 4 years. Forty-seven patients (56.6%) had an isolated PFO, 32 (38.6%) a PFO+IASA, and four (4.8%) an IASA alone. Twenty-six patients (31.3%) had at least one coagulation abnormality detected on the initial thrombophilia screening, with 18 patients (21.7%) exhibiting more than one abnormality. The most important abnormalities which lead to treatment changes in 11 patients (13.3%) were primary anti-phospholipid syndrome (n=3, 3.6%), protein S deficiency (n=2, 2.4%), and hyperhomocysteinemia (n=6/72 screened, 8.3%). At long-term follow-up, seven patients (8.5%) had recurrent TIA (n=6) or ischemic stroke (n=1) in association with a PFO (n=5), or PFO+IASA (n=2). Of the five patients (6.0%) who had recurrent TIAs in association with an isolated PFO, four had potential ‘competing mechanisms’ for their recurrent TIAs: one (1.2%) had a vertebral artery dissection, two (2.4%) had anti-phospholipid syndrome, and one (1.2%) was non-compliant with secondary preventive therapy. This led to an ‘annualized incidence’ of recurrent TIA or stroke of 2.1%/year in the entire cohort, and 0.6%/year in patients in whom no other etiology was identified for their recurrent events other than the presence of a PFO/IASA. Four patients (4.8%) had PFO closure at 2, 12, 13, and 36 months after symptom onset. Two had protein S deficiency, but neither had recurrent TIA or stroke before or after PFO closure. The other two patients who underwent PFO closure had no identifiable thrombophilia. Overall, these data facilitated optimized secondary prevention treatment with changes in the treatment in 13% of the studied population and in 61% of the individuals who had an underlying thrombophilia. Taking together past and new evidence on types of thrombophilia and ideal protocolization for proper screening, Table 1 summarizes the ideal method and timing for thrombophilia screening with its corresponding supporting evidence focused in PFO patients.
The first contemporary effort to clarify the role of transcatheter PFO closure among patients with thrombophilia was performed by Hviid et al. [5] In this systematic review and meta-analysis, 11 PFO closure studies (2,382 patients) were evaluated to determine the recurrence rates among patients with or without thrombophilia who had had a PFO-related cryptogenic stroke. A total of 670 patients with thrombophilia and a PFO-related neurological event were identified and compared to 1,712 controls without thrombophilia. After a mean follow-up of 3.5 years, there were a total of 208 recurrent events. The overall risk of recurrent events among patients with either acquired or inherited thrombophilia and PFO was higher compared to patients with PFO and no thrombophilia (OR, 2.41; 95% CI, 1.44 to 4.06). Stratifying the meta-analysis according to secondary prophylaxis resulted in a higher risk among thrombophilia patients treated by antithrombotic therapy alone (OR, 2.83; 95% CI, 1.41 to 5.68), while the pooled risk estimates in thrombophilia patients treated with PFO closure was not significant (OR, 2.07; 95% CI, 0.95 to 4.48). It should be highlighted that there was a large variability across studies regarding the type of thrombophilia abnormality tested as well as the antithrombotic regimens for secondary prophylaxis.
Prospective comparisons of transcatheter PFO closure in patients with or without thrombophilia were lacking until the recent results from Liu et al. [9] (Table 2). From January 2005 to March 2018, 591 patients diagnosed with PFO-attributable cryptogenic embolism were prospectively enrolled. Patients with a history of oral contraceptive use, active cancer, and other diseases that may have effects on thrombophilia tests were excluded. The authors routinely evaluated all previously known- or thought to be known-PFO related thrombophilias (protein C and S, antithrombin III, factor V Leiden, homocysteine, the anticardiolipin antibodies, lupus anticoagulant, prothrombin G20210A mutation, and factor VIII levels [when the functional assay for protein S was decreased]) and confirmed the results at 3 months in some pre-specified types of thrombophilia. After a weekly “PFO committee” and after a complete evaluation of each case, patients were designated to PFO closure+medical treatment or just medical treatment. After PFO closure, patients received aspirin (81 or 325 mg/day) and/or clopidogrel (75 mg/day) at the discretion of the operator. Patients who had thrombophilia and a single embolism were anticoagulated with warfarin for 3 months with a target international normalized ratio between 2 and 3 and then switched to aspirin. Patients with two or more embolic events were anticoagulated with lifelong warfarin therapy. The primary endpoint was the long-term composite of recurrence of TIA or stroke. After a median follow-up of 53 months, 134 patients (22.7%) were identified with at least one thrombophilia abnormality. The main outcome occurred in 21 patients (15.7%) among those patients with thrombophilia and in 38 patients (8.3%) among those without thrombophilia (HR thrombophilia vs. without thrombophilia: 1.85; 95% CI, 1.09 to 3.16; P=0.024). From those patients with thrombophilia, 88 patients underwent PFO closure with a 100% success rate, whereas 46 patients received medical therapy only. Of the 46 patients who received medical therapy only, 31 (67.4%) received anticoagulation therapy, including 14 patients who received short-term therapy (<3 months) and 17 patients who received lifelong therapy, and 15 patients received antiplatelet therapy. The primary endpoint occurred in six patients (6.7%) in the PFO closure group and in 15 patients (33.3%) in the medical therapy group (HR for PFO closure vs. medical therapy: 0.23; 95% CI, 0.09 to 0.61; P=0.003) (Figure 2). When the individual components of the primary endpoint were analyzed, stroke occurred in one patient (1.1%) in the closure group and in six patients (13.3%) in the medical therapy group (HR, 0.09; 95% CI, 0.01 to 0.77; P=0.028), whereas TIA events occurred in five (5.6%) and nine (20.0%) patients, respectively (HR, 0.33; 95% CI, 0.11 to 1.00; P=0.051). These results were similar after multivariate Cox regression analysis. The almost 3-fold higher rate of recurrent events among thrombophilia patients would support comprehensive hypercoagulability testing in patients with PFO-attributable cryptogenic embolism, particularly considering the reduction of 78% in the risk for embolic events with PFO closure among these patients, even after adjustments for possible confounding factors (such as age, sex, traditional risk factors [hypertension, diabetes, hypercholesterolemia, and smoking history], and PFO anatomical characteristics [moderate to large shunt size and ASA]). Among patients with thrombophilia from the medical therapy group, the treatment with anticoagulation showed a beneficial trend compared with antiplatelet therapy (22.6% vs. 53.3%, P=0.080) in reducing recurrent events; nevertheless, there was no information regarding bleeding events among these patients or among the patients who underwent PFO closure.
Finally, the most recent evidence in the field came from a retrospective registry from Ben-Assa et al. [10] (Table 2), which evaluated 800 consecutive patients undergoing PFO closure. After an exhaustive hypercoagulability testing of all patients with confirmatory protocols, 239 (29.9%) patients were found to have a hypercoagulable disorder. A treatment of at least 3 months of anticoagulation following PFO closure was used in patients with thrombophilia. Follow-up events included death, recurrent neurological events, and the need for reintervention for a significant residual shunt. After a median follow-up of 42 months, there were no differences in the rate of stroke or TIA between patients with or without thrombophilia (2.5% in non-hypercoagulable group vs. 3.4% in hypercoagulable group, log-rank test P=0.35).
Recent evidence suggested that in some high thromboembolic risk scenarios, PFO closure may be superior to medical therapy for primary prevention of neurological events. In a single-center retrospective study [11], 511 consecutive patients with PFO but without prior history of stroke were systematically screened for a HCS finding, and 136 patients were maintained on lifetime antithrombotic therapy based on the presence of a “significant clinical manifestation” or a family history of such (history of or presence of deep vein thrombosis, presence of collagen vascular disease, recurrent abortions, of a first-degree relative diagnosed with a clinically significant HCS). These patients, who were allocated to PFO closure versus medical therapy in a non-randomized fashion after medical team discussions (which included the cardiology and hematology teams, who performed individual analysis and treatment allocation based on the agreement of both sides) were followed-up for the primary outcome of stroke or TIA. The medical therapy allocation consisted of continuing their already indicated medical treatment, which was OAC and antiplatelet therapy in 72% and 22% of patients, respectively. Transcatheter PFO closure was performed in 85 (63%) patients and antithrombotic therapy was not interrupted prior to or after the procedure.
At a mean follow-up of 46 months, 23 (17%) patients experienced an outcome event (16 patients, 31% of the non-PFO closure group vs. seven patients, 8% of the PFO closure group) (Figure 3). In a multivariate Cox proportional hazards regression analysis, PFO closure was independently associated with a lower risk HR of stroke/TIA occurrence with a HR of 0.18 (95% CI, 0.03 to 0.3; P=0.001).
The recommended antithrombotic treatment following transcatheter PFO closure in the general population is based on the most recent ESC clinical guidelines [7], which recommended dual antiplatelet therapy for 1 to 6 months following the procedure and single antiplatelet therapy for at least 5 years thereafter. There are no specific recommendations on antithrombotic treatment following PFO closure in patients with thrombophilia. In previous studies on transcatheter PFO closure in thrombophilia patients, medical treatment consisted mainly of OAC, particularly warfarin (with or without aspirin), ranging from 3 months to lifelong treatment according to the number of previous thrombotic events and/or the specific type of thrombophilia. An international normalized ratio between 2 and 3 was targeted in all studies (Table 4). While waiting for definite data, available clinical data would support a strategy of OAC with warfarin following PFO closure, with tailored treatment duration according to the number of events and specific types of thrombophilia. The final decision should likely be individualized after a discussion including cardiologists and hematologists. The role of other novel anticoagulation therapies in this population remains unknown.
In conclusion, new evidence strongly suggests that stroke patients with PFO should be systematically screened for thrombophilia in order to identify those who are at higher risk and may benefit from transcatheter PFO closure, alongside optimal antithrombotic management, which remains to be determined in such a complex population. Transcatheter PFO closure in patients with thrombophilia is as safe and effective as in patients without thrombophilia, with very low complication rates and comparable long-term clinical outcomes, and a low rate of recurrent neurological events. If PFO closure should be proposed as the primary prevention of PFO-associated stroke remains to be determined, and this should be properly addressed in prospective randomized trials.
Notes
Disclosure
Josep Rodés-Cabau has received institutional research grants from Abbott Vascular Canada, and holds the Research Chair Fondation "Famille Jacques Larivière" for the Development of Structural Heart Disease Interventions. The rest of authors do not disclose any potential conflict of interest with respect to the content of this study.
Figure 1.
Recurrent cerebral ischemic events before transcatheter patent foramen ovale (PFO) closure in patients with or without thrombophilia. Event-free rate and 95% confidence intervals (dashed lines) of recurrent cerebral ischemia before percutaneous PFO closure in patients who did (squares) and those who did not have an associated thrombophilia (triangles). Reproduced from Giardini et al. [6], with permission from Elsevier.
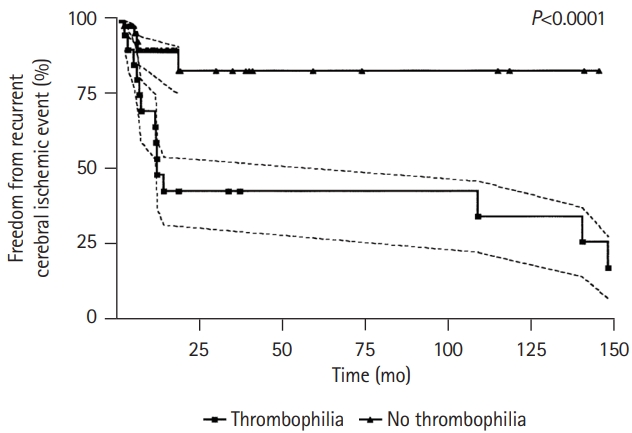
Figure 2.
Rate of primary endpoints in patients with or without thrombophilia according to their treatment allocation after a patent foramen ovale (PFO)-related cerebrovascular event. Kaplan-Meier cumulative estimates demonstrate that PFO closure significantly reduced recurrent events of stroke or transient ischemic attack compared with medical therapy in patients with thrombophilia to a greater extent than medical therapy alone. Reproduced with permission from Liu et al [9].
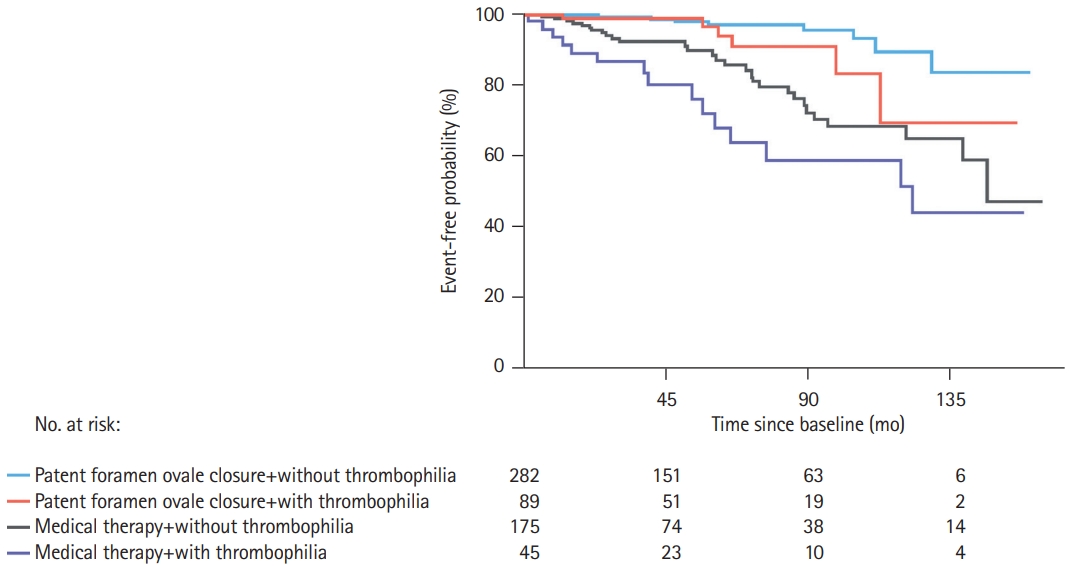
Figure 3.
Long-term outcomes of patients with or without hypercoagulable state after patent foramen ovale (PFO) closure as primary prevention strategy before a left systemic cerebrovascular event. Kaplan-Meier estimates of survival free of neurological events in patients with significant hypercoagulable state according to closure of PFO. Dash line represents patients who underwent PFO closure whereas the continuous line represents patients who received only medical treatment. Reproduced from Buber et al. [11], with permission from Karger Publishers. CVA, cerebrovascular accident; TIA, transient ischemic attack.
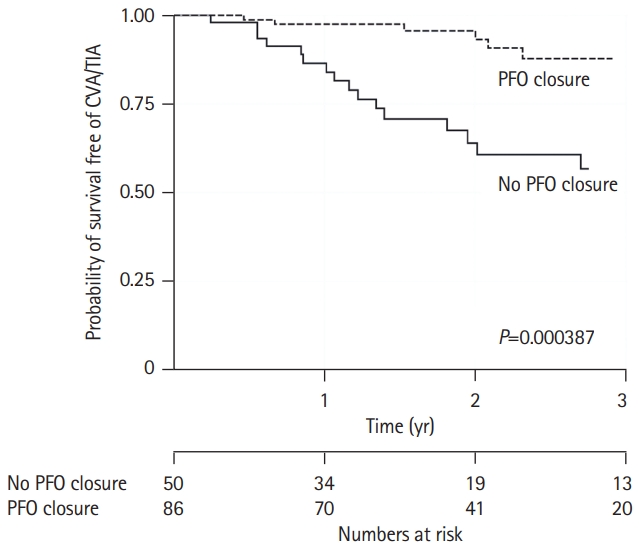
Table 1.
Thrombophilia screening in patients with a PFO-related thromboembolic event
| Prevalence in general population (%) | Risk for thromboembolic event in general population | Confirmatory test required* | Supporting evidence in PFO patients | ||
|---|---|---|---|---|---|
| Venous hypercoagulability | |||||
| Factor V Leiden† | 4-104 | 5-10-fold (heterozygous)4 | No | [14-25] | |
| 50-100-fold (homozygous)4 | |||||
| Prothrombin G2010A mutation | 1-54 | 3-5-fold (heterozygous)4 | No | [14-16,18-25] | |
| Protein C deficiency‡ | 0.2-0.54 | 6.5-8-fold4 | Yes | [19-22,24] | |
| Protein S deficiency‡ | 0.74 | 1.6-11.5-fold4 | Yes | [10,17,19-22,24] | |
| Antithrombin III deficiency§ | 0.174 | 5-8.1-fold4 | Yes | [19-21] | |
| Increased factor VIII activity∥,¶ | 4.44 | 0.3-1.8-fold2 | Yes | [10,20] | |
| Elevated lipoprotein (a) | 204 | 1.6-2.2-fold26 | Yes | [21] | |
| Mixed hypercoagulability | |||||
| Methylenetetrahydrofolate reductase (MTHFR) mutation**,†† | 113 | 3-fold4 | No | [10,19-22,24,25] | |
| Antiphospholipid syndrome‡‡ | 1-5425 | 0.6-10-fold27 | Yes | [10,19-25,28] | |
The timing for screening depends on the type of test and the timing of the clinical event. For genetic mutations, the test can be performed at any time, but for functional or antibody tests, these should be ideally performed 1 to 2 months after the thrombotic clinical event to avoid false results in the initial test.
PFO, patent foramen ovale.
‡ Can be inherited or acquired. Acquired common causes can be malignancy, liver disease, warfarin or vitamin K deficiency, among others;
§ Can be inherited or acquired. Acquired common causes can be liver disease, nephrotic syndrome, among others;
¶ Can be inherited or acquired. Acquired causes are pregnancy, malignancies, infection and inflammation;
Table 2.
Transcatheter PFO closure after a PFO-related cerebrovascular event in patients with thrombophilia: safety, efficacy, and long-term clinical outcomes
| Study | Study design* | Study population | PFO closure indication | Implanted devices & PFOc success rate | Clinical follow-up | Remarks | |
|---|---|---|---|---|---|---|---|
| Giardini et al. (2004) [6] | Prospective, comparative, non-randomized | 72 PFO patients screened: | ≥1 documented stroke (51%) or TIA (49%) of unknown origin | Cardioseal STARFLEX® | Median FU: 19 months | No thrombus on the device at 6-months FU TOE. Before closure, patients with thrombophilia had a higher rate of recurrent events that patients without it. | |
| PFOc in patients with vs. without thrombophilia | 20 patients (28%) with thrombophilia | Amplatzer PFO occluder® | Primary endpoint: recurrence of TIA or stroke, 4% of overall population without differences among groups | ||||
| No cancer patients were included | 99% | ||||||
| Kar et al. (2017) [31] | Retrospective analysis | 861 PFO patients screened: | Stroke or TIA (70.7%) | Amplatzer PFO occluder® | Median FU: 43 months | None of the GORE® Helex (33 implants) developed thrombus formation. | |
| PFOc in patients with reversible or irreversible thrombophilias | 142 (16.5%) with any kind of thrombophilia | Migraine (20.4%) | Amplatzer Cribriform occluder® | One patient (1.4%) in the irreversible thrombophilia group had a recurrent stroke after PFOc. | |||
| Peripheral embolism (3.4%) | |||||||
| 46.9% underwent PFOc | Right ventricular enlargement (1.4%) | GORE® Helex | |||||
| 2.7% had cancer as thrombotic state | Desaturation (0.7%) | 100% | |||||
| Combination (3.4%) | |||||||
| Liu et al. (2020) [9] | Prospective, comparative, non-randomized | 591 Patients screened: | Stroke (81%) or TIA (19%) | Not specified | Median FU: 54 months | ||
| PFOc vs. MT in patients with PFO and thrombophilia | 134 patients (22.7%) with thrombophilia: | 100% | Primary endpoint: recurrence of TIA or stroke, PFOc 6 patients (6.7%) vs. MT 15 patients (33.3%) (HR, 0.23; 95% CI, 0.09-0.61; P=0.003) | ||||
| - 88 to PFOc | |||||||
| - 46 to MT | |||||||
| No cancer patients were included | |||||||
| Ben-Assa et al. (2021) [10] | Retrospective, comparative | 800 PFO patients screened: | Stroke (69.9%) TIA (14.6%) | Amplatzer PFO occluder® | Median FU: 41 months | 1.3% Recurrent stroke rate in the thrombophilic group. Included high levels of lipoprotein (a) as hypercoagulable state (32.6% of this cohort). | |
| PFOc in patients with vs. without thrombophilia | 239 patients (29.9%) with thrombophilia. Cancer was not considered a thrombotic stat nor an exclusion criterion | Multiple cerebrovascular events (10%) | Cardioseal STARFLEX® | Primary endpoint: recurrence of TIA or stroke, thrombophilia group 3.4% vs. 2.5% in the group without it (P=0.35) | |||
| Hypoxemia (0.8%) | GORE® | ||||||
| Peripheral embolism (2.9%) | 99.20% | ||||||
| Migraine (1.7%) | |||||||
Table 3.
Summary of recommendations of current clinical management guidelines on PFO-related stroke focused on patients with thrombophilia
Table 4.
Medical treatment after transcatheter PFO closure for patients with thrombophilia in recent trials
| Study | Treatment in groups without thrombophilia | Treatment in groups with thrombophilia | Remarks |
|---|---|---|---|
| Giardini et al. (2004) [6] | Aspirin 100 mg/day for the first 6 months and ticlopidine 250 mg b.i.d. for the first 3 months | Warfarin first 6 months | Routine monitoring with complete blood cell count at 30 days, 3 months, 6 months |
| Target INR between 2 and 3 | |||
| Liu et al. (2020) [9] | Aspirin 81 or 325 mg/day and/or clopidogrel 75 mg/day | Single embolic event: Warfarin for 3 months | Target INR between 2 and 3 |
| Duration not specified. | Two or more embolic events: Lifelong warfarin | Choice of medical therapy in group without thrombophilia was left at the discretion of the operator. | |
| Ben-Assa et al. (2021) [10] | Aspirin 325 mg/day or aspirin 100 mg/day+clopidogrel 75/day for 3 months, then switched to aspirin 100/day thereafter | Patients with non-arterial HCS+1 episode of provoked thrombotic event*: 3 months of warfarin and switched to aspirin 100 mg/day thereafter | Non-arterial HCS: antithrombin III, protein C, or protein S deficiency, or who were carriers of the factor V Leiden or prothrombin G20210A mutation |
| Same patients but with ≥2 episodes of thrombotic events OR any patient with an arterial HCS: life-long warfarin anticoagulation | Arterial HCS: anticardiolipin antibody, lupus anticoagulant, or hyper-homocysteinemia |
References
1. Giacoppo D, Caronna N, Frangieh AH, Michel J, Andò G, Tarantini G, et al. Long-term effectiveness and safety of transcatheter closure of patent foramen ovale compared with antithrombotic therapy alone: a meta-analysis of six randomised clinical trials and 3,560 patients with reconstructed time-to-event data. EuroIntervention 2018;14:857-867.


2. Salehi Omran S, Hartman A, Zakai NA, Navi BB. Thrombophilia testing after ischemic stroke: why, when, and what? Stroke 2021;52:1874-1884.


3. Thrombophilia and their detection. In : Wahed A, Quesada A, Dasgupta A, editors. Hematology and Coagulation 2nd ed. Amsterdam (NL): Academic Press; 2020. p. 265-276.
4. Mac Grory B, Ohman EM, Feng W, Xian Y, Yaghi S, Kamel H, et al. Advances in the management of cardioembolic stroke associated with patent foramen ovale. BMJ 2022;376:e063161.

5. Hviid CV, Simonsen CZ, Hvas AM. Recurrence risk in patients with cryptogenic stroke, patent foramen ovale, and thrombophilia: a systematic review and meta-analysis. Thromb Haemost 2019;119:1839-1848.


6. Giardini A, Donti A, Formigari R, Bronzetti G, Prandstraller D, Bonvicini M, et al. Comparison of results of percutaneous closure of patent foramen ovale for paradoxical embolism in patients with versus without thrombophilia. Am J Cardiol 2004;94:1012-1016.


7. Pristipino C, Sievert H, D’Ascenzo F, Louis Mas J, Meier B, Scacciatella P, et al. European position paper on the management of patients with patent foramen ovale: general approach and left circulation thromboembolism. Eur Heart J 2019;40:3182-3195.


8. Messé SR, Gronseth GS, Kent DM, Kizer JR, Homma S, Rosterman L, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the Guideline Subcommittee of the American Academy of Neurology. Neurology 2020;94:876-885.



9. Liu K, Song B, Palacios IF, Inglessis-Azuaje I, Deng W, McMullin D, et al. Patent foramen ovale attributable cryptogenic embolism with thrombophilia has higher risk for recurrence and responds to closure. JACC Cardiovasc Interv 2020;13:2745-2752.



10. Ben-Assa E, Herrero-Garibi J, Cruz-Gonzalez I, Elmariah S, Rengifo-Moreno P, Al-Bawardy R, et al. Efficacy and safety of percutaneous patent foramen ovale closure in patients with a hypercoagulable disorder. Catheter Cardiovasc Interv 2021;98:800-807.



11. Buber J, Guetta V, Orion D, Lubetsky A, Borik S, Vatury O, et al. Patent foramen ovale closure among patients with hypercoagulable states maintained on antithrombotic therapy. Cardiology 2021;146:375-383.



12. Kalaria C, Kittner S. The therapeutic value of laboratory testing for hypercoagulable states in secondary stroke prevention. Neurol Clin 2015;33:501-513.


13. Arachchillage DJ, Mackillop L, Chandratheva A, Motawani J, MacCallum P, Laffan M. Thrombophilia testing: a British Society for Haematology guideline. Br J Haematol 2022;198:443-458.




14. Lim ST, Murphy SJ, Smith DR, Williams J, Navarro SG, McCabe J, et al. Clinical outcomes and a high prevalence of abnormalities on comprehensive arterial and venous thrombophilia screening in TIA or ischaemic stroke patients with a patent foramen ovale, an inter-atrial septal aneurysm or both. J Neurol Sci 2017;377:227-233.


15. Elgendy AY, Saver JL, Amin Z, Boudoulas KD, Carroll JD, Elgendy IY, et al. Proposal for updated nomenclature and classification of potential causative mechanism in patent foramen ovale-associated stroke. JAMA Neurol 2020;77:878-886.


16. Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009;40:2349-2355.



17. Pezzini A, Del Zotto E, Magoni M, Costa A, Archetti S, Grassi M, et al. Inherited thrombophilic disorders in young adults with ischemic stroke and patent foramen ovale. Stroke 2003;34:28-33.


18. Karttunen V, Hiltunen L, Rasi V, Vahtera E, Hillbom M. Factor V Leiden and prothrombin gene mutation may predispose to paradoxical embolism in subjects with patent foramen ovale. Blood Coagul Fibrinolysis 2003;14:261-268.


19. Ford MA, Reeder GS, Lennon RJ, Brown RD, Petty GW, Cabalka AK, et al. Percutaneous device closure of patent foramen ovale in patients with presumed cryptogenic stroke or transient ischemic attack: the Mayo Clinic experience. JACC Cardiovasc Interv 2009;2:404-411.

20. Pezzini A, Grassi M, Zotto ED, Giossi A, Volonghi I, Costa P, et al. Do common prothrombotic mutations influence the risk of cerebral ischaemia in patients with patent foramen ovale?: systematic review and meta-analysis. Thromb Haemost 2009;101:813-817.


21. Debski M, Abdelrahman A, Alshehri H, Prince M, Wiper A, Chalil S, et al. Contemporary management of patent foramen ovale: a multinational survey on cardiologists’ perspective. J Interv Cardiol 2021;2021:6955791.


22. Omran SS, Lerario MP, Gialdini G, Merkler AE, Moya A, Chen ML, et al. Clinical impact of thrombophilia screening in young adults with ischemic stroke. J Stroke Cerebrovasc Dis 2019;28:882-889.



23. Offelli P, Zanchetta M, Pedon L, Marzot F, Cucchini U, Pegoraro C, et al. Thrombophilia in young patients with cryptogenic stroke and patent foramen ovale (PFO). Thromb Haemost 2007;98:906-907.


24. Kefer J, Sluysmans T, Hermans C, El Khoury R, Lambert C, Van de Wyngaert F, et al. Percutaneous transcatheter closure of interatrial septal defect in adults: procedural outcome and long-term results. Catheter Cardiovasc Interv 2012;79:322-330.


25. Giardini A, Donti A, Formigari R, Salomone L, Palareti G, Guidetti D, et al. Spontaneous large right-to-left shunt and migraine headache with aura are risk factors for recurrent stroke in patients with a patent foramen ovale. Int J Cardiol 2007;120:357-362.


26. Ding WY, Protty MB, Davies IG, Lip GY. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc Res 2022;118:716-731.




27. Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003;101:1827-1832.



28. Dodge SM, Hassell K, Anderson CA, Keller J, Groves B, Carroll JD. Antiphospholipid antibodies are common in patients referred for percutaneous patent foramen ovale closure. Catheter Cardiovasc Interv 2004;61:123-127.


29. Yan C, Li H. Preliminary investigation of in situ thrombus within patent foramen ovale in patients with and without stroke. JAMA 2021;325:2116-2118.











