Dear Sir:
Systems of care for emergent large vessel occlusion (ELVO) stroke patients have evolved from a sole focus on intravenous thrombolysis (IVT) to also incorporate timely access to endovascular thrombectomy (EVT) [1,2]. Prehospital Emergency Medical Services (EMS) triage has historically transported these patients to the closest stroke center for IVT, regardless of stroke center classification, with a subsequent transfer if the initial facility was not a Thrombectomy Capable Stroke Center (TSC). Triage of ELVO stroke patients directly to TSCs, possibly bypassing primary stroke centers (PSCs), has been modeled to improve treatment times and access to EVT [3]. These protocols have led to higher rates of EVT, improved time to EVT without delays to IVT, and potentially less disability at 3 months [4-6]. Our objective is to determine whether an updated New York City (NYC) EMS Acute Stroke Triage Protocol reduced the proportion of ELVO stroke patients requiring interfacility transfers and time to EVT.
This is a retrospective observational study using prospectively collected data (IRB-20-03285) at our health system (Mount Sinai Health System, New York, NY, USA), which consists of six emergency departments with three additional covered through affiliate agreements. Written informed consent by the patients was waived due to the retrospective nature of our study. Of these nine hospitals, four were TSCs (Supplementary Figure 1). These four TSCs are a subset of the 22 total TSCs across NYC participating in the updated NYC EMS Acute Stroke Triage Protocol enacted in April 2019 (Supplementary Figure 2). The protocol (Figure 1) was developed by the NYC Regional Emergency Medical Advisory Committee, NYC Fire Department, Greater New York Hospital Association, and American Heart Association [7]. It utilizes the Los Angeles Motor Scale with the addition of Speech (S-LAMS). This scale was chosen for ease of training EMS personnel and accuracy of detecting ELVO strokes, as compared to LAMS without Speech [7,8]. Direct medical oversight (DMO) is required and consists of emergency physicians trained in oversight of EMS with specific training in neurological emergencies. They are equipped with TSC availability and EMS medical records. Prior to the updated protocol, stroke patients were transported to the closest stroke center, regardless of classification, without contacting DMO. As with most high acuity conditions, there was still a pre-notification to the emergency department from EMS and the Cincinnati Prehospital Stroke Scale was used to communicate exam findings.
The study population included all ELVO stroke patients who underwent EVT between April 2018 to March 2020. Other inclusion criteria included a last known well (LKW)-to-EMS Arrival Ōēż5 hours. Exclusion criteria were inpatient status at time of stroke, non-EMS transport, or unknown LKW or EMS Arrival times. The primary endpoint was the proportion of ELVO stroke patients requiring an interfacility transfer for EVT and secondary endpoints were the time from EMS arrival to EMS left scene, initial hospital arrival, IVT, TSC arrival, and EVT access (Supplementary Methods and Appendix 1).
Among 415 EVT cases, 193 patients were included and separated into pre- (April 2018 to March 2019; n=97) and post-protocol (April 2019 to March 2020; n=96) cohorts (Supplementary Figure 3). The cohorts were similar in age, sex, and past medical history, except for a higher proportion with atrial fibrillation in the pre-protocol cohort (46.4% vs. 31.3%, P=0.04). The median admission National Institutes of Health Stroke Scale was similar but the pre-stroke modified Rankin Scale was less in the post-protocol cohort (0.0 [2.0] vs. 0.0 [1.5], P=0.02). The breakdown of ELVO locations was different and LKW-to-EMS arrival and procedural details were similar. In the post-protocol cohort 28.1% were triaged as positive (Supplementary Table 1).
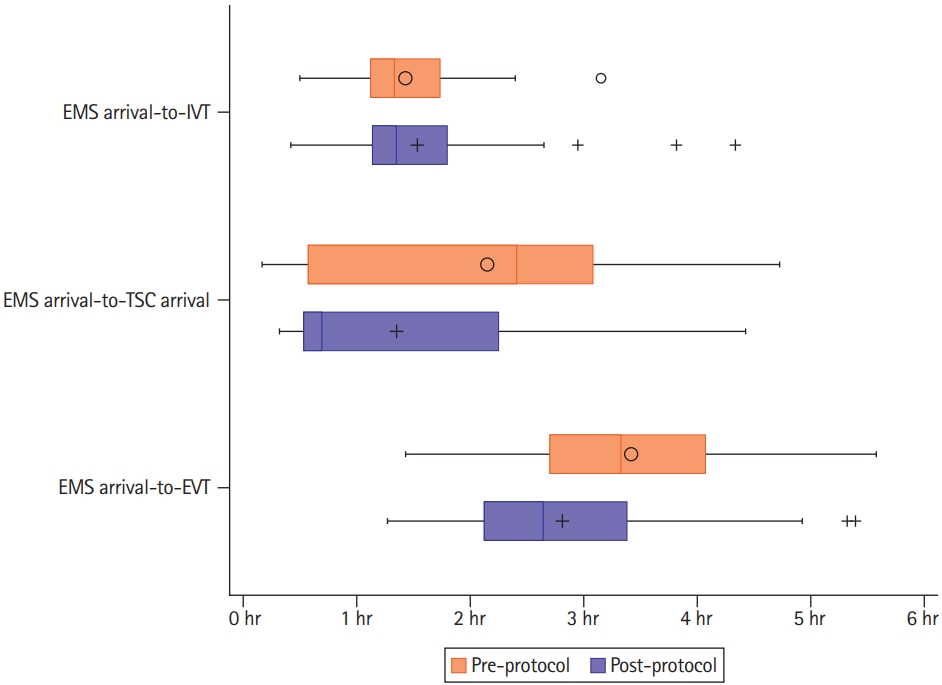
The primary endpoint, the proportion of ELVO stroke patients requiring an interfacility transfer, was significantly reduced following the implementation of the updated protocol (63.9% vs. 37.5%, P<0.01). The median EMS arrival-to-left scene (20.0 minutes vs. 24.0 minutes, P=0.02) and EMS arrival-to-initial hospital arrival (28.0 minutes vs. 33.5 minutes, P<0.01) were significantly longer post-protocol implementation but EMS arrival-to-IVT was similar (80.5 minutes vs. 81.5 minutes, P=0.42). The median EMS arrival-to-TSC arrival (150.0 minutes vs. 44.5 minutes, P<0.01) and EMS arrival-to-EVT access (209.0 minutes vs. 164.0 minutes, P<0.01) were both significantly faster post-protocol implementation (Table 1 and Figure 2).
We know from the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) and Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registries that ELVO stroke patients requiring an interfacility transfer had significant delays to EVT, translating to worse outcomes at 3 months [9,10]. The updated protocol significantly reduced interfacility transfers and EMS arrival-to-EVT, similar to another high population density region, Stockholm, Sweden, which decreased interfacility transfers from 76% to 27% (P<0.01) and saved 69 minutes from LWK-to-EVT (206.0 vs. 137.0, P<0.01) [4].
Post protocol implementation, 28.1% of patients were triaged as positive and 37.5% required an interfacility transfer for EVT. Notably, none of the transferred patients were triaged as positive using the updated protocol (52.2% in this cohort who were not triaged as positive required interfacility transfer). Possible causes may be proper protocol adherence, protocol exclusion criteria, and unpredictable wait times from DMO. Per the protocol, DMO approval is required for direct transport to a TSC (even if it is the closer than a PSC). Therefore, EMS may not be able to follow the protocol if the DMO wait time is long. This may have led to the prolonged on-scene time interval (although this did not adversely impact treatment times) and a spillover effect of transporting patients directly to TSCs without DMO approval (hence the relatively low positive triage).
In a prior study, when a speech abnormality was added to a LAMS of 3, there was a sensitivity, specificity, and negative predictive value of 61%, 70%, and 90%, respectively, for detection of ELVO strokes [8]. This was similar to the Rapid Arterial Occlusion Evaluation (RACE) and an improvement upon LAMS (without speech), Cincinnati Prehospital Stroke Severity Scale, and 3-Item Stroke Scale [11]. A limitation of our study was the limited population of only ELVO stroke patients who underwent EVT.
The updated NYC EMS Acute Stroke Triage Protocol reduces the proportion of ELVO stroke patients requiring interfacility transfer for EVT, decreases time to EVT, and does not delay time to IVT. This study provides preliminary evidence that may be used for other regions to individualize triage protocols to decrease time to EVT in areas with TSC availability within a reasonable travel time.