 |
 |
- Search
| J Stroke > Volume 23(2); 2021 > Article |
|
Dear Sir:
Recent randomized trials have shown the benefit of patent foramen ovale (PFO) closure for secondary stroke prevention in patients with high-risk PFO [1-3]. Because these trials enrolled relatively young (<60 years) patients, the current American Academy of Neurology guidelines recommend PFO closure in stroke patients who are <60 years old [4]. Therefore, the benefit of PFO closure in older patients remains unclear [5]. Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients With High-Risk Patent Foramen Ovale (DEFENSE-PFO) was a randomized trial that showed that cardio-cerebral vascular events were significantly reduced in the PFO-closure arm than in the medical-treatment arm [6]. Since there was no age limit in this study, we aimed to investigate the benefit of PFO closure in older adults. The DEFENSE-PFO trial has been described elsewhere [6]. Briefly, we enrolled patients who had an ischemic stroke within the previous six months and a high-risk PFO (PFO with an atrial septal aneurysm, hypermobility, or size of ≥2 mm on transesophageal echocardiography) with no other identifiable causes. Patients with significant (≥50%) cerebral artery steno-occlusion or lacunar infarcts were excluded. The trial was approved by the Institutional Review Board at participating centers, and all patients provided written informed consent.
For this study, patients were grouped into two (<60 and ≥60 years old). The primary endpoint was ischemic stroke or transient ischemic attack (TIA) during 2 years of follow-up. The secondary endpoints included stroke, TIA, vascular death, and Thrombolysis in Myocardial Infarction (TIMI)-defined major bleeding. The baseline characteristics were compared using Pearson’s chi-square tests or Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous variables, as indicated. All analyses were conducted using the intentionto- treat population. Cumulative incidence was plotted using Kaplan-Meier curves and compared using the log-rank test. The hazard ratio (HR) was evaluated using the penalized maximum likelihood estimation. The interactions between the treatment allocation and age subgroups for each endpoint were evaluated using the Cox proportional hazard model. Considering the small sample of patients with advanced age and the explorative nature of the study, we set the significant P-value level as 0.1 rather than 0.05.
From September 2011 to October 2017, 120 patients (mean age, 52 years; 60 patients in each arm) were enrolled. There were 86 (71.7%) and 34 (28.3%) patients in the <60 and ≥60 years groups, respectively. Patients in the ≥60 years group had a higher prevalence of cardiovascular risk factors and lower paradoxical embolism (risk of paradoxical embolism [RoPE]) scores than those in the <60 group years (Supplementary Table 1). In the <60 and ≥60 years groups, 47 (54.7%) and 13 (38.2%) patients were assigned to the PFO-closure arm, respectively. The baseline characteristics of the patients are summarized in Table 1. The baseline characteristics and anatomic features of PFO were similar in both groups, except for the higher RoPE score (7.6±1.5 vs. 6.9±1.2, P=0.02) and fewer current smokers (17.0% vs. 35.9%, P=0.05) in the PFO-closure arm of the <60 years group.
The median follow-up duration was 2.5 years (interquartile range [IQR], 0.8 to 3.9) for the <60 years and PFO closure group, 2.9 years (IQR, 0.9 to 4.6) for the <60 years and medical treatment group, 4.4 years (IQR, 3.7 to 5.0) for the ≥60 years and PFO closure group, and 2.5 years (IQR, 0.8 to 3.9) for the ≥60 years and medical treatment group. Recurrent ischemic stroke or TIA occurred in six patients; all were from the medical therapy arm (Table 2). The Kaplan-Meier curve showed no difference (2-year event rate, 5.8%; HR, 5.99; 95% confidence interval [CI], 0.15 to 246.68; log-rank P=0.12) in the <60 years group; it showed a difference (2-year event rate, 24.6%; HR, 7.36; 95% CI, 0.28 to 195.81; log-rank P=0.07) between the two treatment arms in the ≥60 years group (Figure 1). P-value for interaction was 0.96. When the ages of the patients were ≥70 years, the incidence of the primary outcome was significantly higher in the medication group than in the PFO closure group (2-year event rate, 80%; HR, 11.64; 95% CI, 0.43 to 318.81; log-rank P=0.03) (Supplementary Table 2 and Supplementary Figure 1). The magnetic resonance imaging (MRI) findings for five patients with recurrent ischemic stroke are summarized in Supplementary Table 3. All recurrent strokes were regarded as embolic infarctions because there was no significant cerebral artery stenosis. Electrocardiography and Holter examinations were performed for all the patients at the time of the initial stroke and repeated for four and two patients, respectively, at the time of recurrence. No embolic causes (e.g., atrial fibrillation) were identified. None of the patients showed MRI findings of lacunar infarction (subcortical infarcts with diameter <20 mm) during the initial and recurrent strokes.
The main DEFENSE-PFO trial showed that PFO closure was effective in preventing recurrent ischemic stroke and some major vascular events [6]. This sub-study indicates that the benefit is greater in patients aged ≥60 years than in younger patients. Further analysis showed that PFO closure was even more effective in patients aged ≥70 years. The interaction analysis, however, did not show significant differences in the primary outcomes across the age groups. Nevertheless, the tendency of a better clinical response to PFO closure in old patients may be attributed to their higher frequency of recurrent strokes regardless of treatment (Supplementary Figure 2). Although PFO size may increase with age [7], there were no significant differences in the PFO size and the prevalence of atrial septal aneurysm and hypermobility in the groups, probably because only patients with high-risk PFO were enrolled in our trial. Thus, morphological PFO characteristics cannot adequately explain our observations. Although vascular risk factors increased with age, all cases of recurrent stroke were regarded as embolic. Previous studies demonstrated that recurrent stroke is common in old patients with PFO [8 9], possibly due to the increased prevalence of venous thrombosis [10] or increased right ventricular pressure that promotes right-to-left shunting [11]. Our findings, therefore, suggest that PFO closure may be needed in older adult patients who are prone to recurrent strokes.
This study has limitations. (1) This subgroup analysis was not prespecified but post hoc. (2) The number of older patients was small; therefore, the results were not adequately powered and may have been a consequence of chance. (3) Although a Holter examination was performed for all patients for the initial stroke, not all patients with recurrent stroke underwent repeated examinations. Despite these limitations, our data suggest that PFO closure may be effective in preventing recurrent stroke in older patients. Although randomized trials involving more patients are required to confirm our preliminary findings, we suggest that PFO closure should be considered seriously, even in older patients.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2021.00647.
Supplementary Table 1.
Baseline characteristics of the patients according to age
Supplementary Table 3.
Magnetic resonance imaging characteristics of stroke in patients with recurrence stroke
Supplementary Figure 1.
Kaplan-Meier estimate for primary endpoint in age ≥70 years group. Event-free survival of the primary endpoint in the patent foramen ovale (PFO)-closure group (red line) versus the medical-treatment group (blue line) (log rank P=0.03).
Supplementary Figure 2.
Kaplan-Meier estimate for primary endpoint according to age. Graph showing primary endpoint event free survival in <60 years group versus ≥60 years group.
Acknowledgments
This work was supported by a research grant from the Cardiovascular Research Foundation, Seoul, South Korea.
Figure 1.
Kaplan-Meier estimates for patent foramen ovale (PFO) closure and primary endpoint. Event-free survival of all the patients in the PFO-closure group (red line), patients aged <60 years in the medical-treatment group (blue line), and patients aged ≥ 60 years in the medical-treatment group (green line).
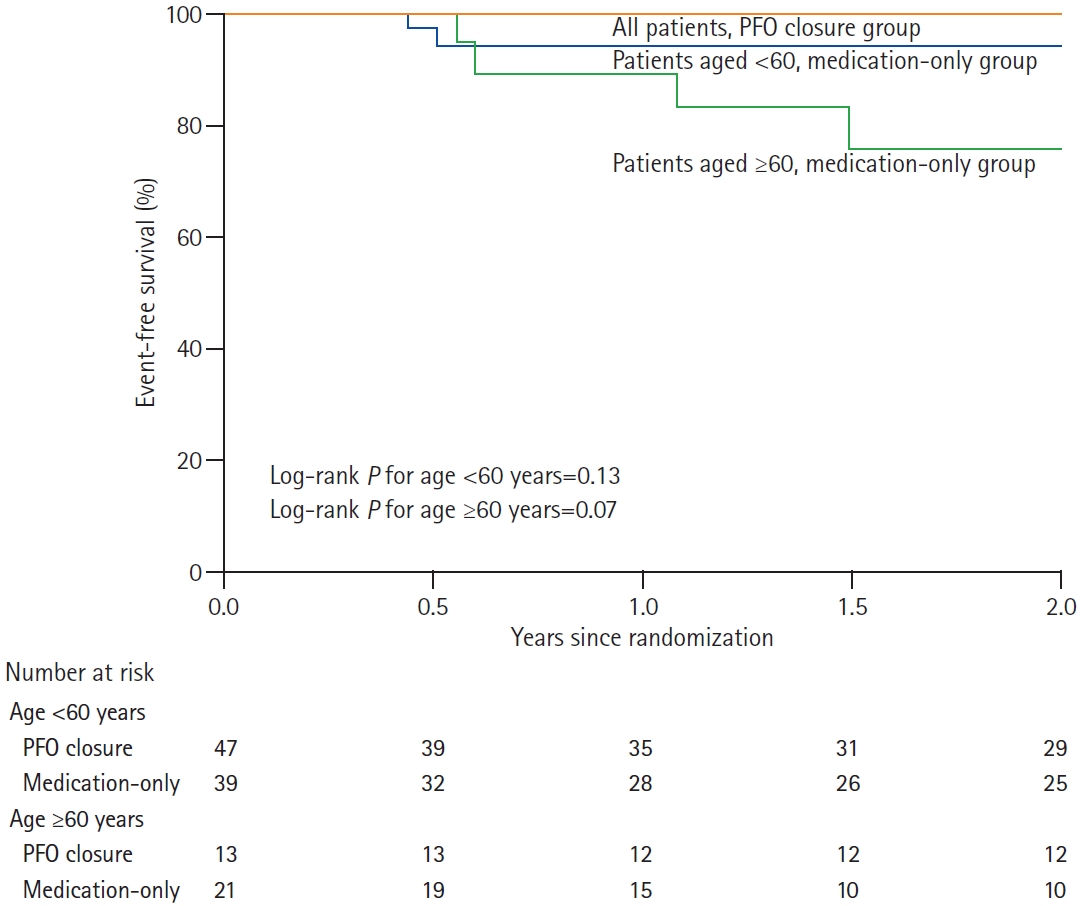
Table 1.
Baseline characteristics of patients aged <60 and ≥60 years
Table 2.
Outcomes of patients aged aged <60 and ≥60 years
References
1. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 2017;377:1022-1032.


2. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med 2017;377:1011-1021.


3. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017;377:1033-1042.


4. Messé SR, Gronseth GS, Kent DM, Kizer JR, Homma S, Rosterman L, et al. Practice advisory update summary: patent foramen ovale and secondary stroke prevention: report of the Guideline Subcommittee of the American Academy of Neurology. Neurology 2020;94:876-885.



5. Köhrmann M, Schellinger PD, Tsivgoulis G, Steiner T. Patent foramen ovale: story closed? J Stroke 2019;21:23-30.



6. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE- PFO trial. J Am Coll Cardiol 2018;71:2335-2342.

7. Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17-20.


8. Homma S, DiTullio MR, Sacco RL, Sciacca RR, Mohr JP; PICSS Investigators. Age as a determinant of adverse events in medically treated cryptogenic stroke patients with patent foramen ovale. Stroke 2004;35:2145-2149.


9. Mazzucco S, Li L, Rothwell PM. Prognosis of cryptogenic stroke with patent foramen ovale at older ages and implications for trials: a population-based study and systematic review. JAMA Neurol 2020;77:1279-1287.


- TOOLS








