 |
 |
- Search
| J Stroke > Volume 22(3); 2020 > Article |
|
This article has been corrected. See "Early Fluid Attenuation Inversion Recovery Sulcal Contrast Enhancement Correlates with Severity of Reversible Cerebral Vasoconstriction Syndrome" in Volume 23 on page 146.
Dear Sir:
Reversible cerebral vasoconstriction syndrome (RCVS) is a relatively newly described neurovascular entity. The clinical outcome is generally benign, but sometimes disabling or life-threatening. Triggers for this condition are variable with a large proportion of idiopathic causes. Several informative papers had been written on this subject [1-3] which include proposals for diagnostic criteria, differentiation from other cerebral vasculopathies, and imaging features.
However, the pathophysiology of the condition is still not well understood, especially in the large proportion of idiopathic cases [3]. A leading hypothesis for the propagation of the condition attributes a significant role to vascular autoregulation disruption similarly to posterior reversible encephalopathy syndrome (PRES) but with different triggers [4]. Early markers of the condition are needed, which would allow prompt treatment, avoid unnecessary studies and shed some light on the RCVS mechanism. Recently a salient study of 23 RCVS patients in South Korea described a phenomenon of contrast enhanced fluid attenuation inversion recovery (CE FLAIR) magnetic resonance imaging (MRI) hyperintensity in cortical sulci interpreted as blood brain barrier (BBB) disruption and showed its correlation to clinical outcome [5]. We observed similar findings in Israeli population, and 18 out of 21 confirmed RCVS patients had exclusively posterior sulcal contrast enhancement (in the posterior occipital, parietal or cerebellar sulci) on CE FLAIR sequences (Figure 1). We also found a positive correlation between the extent of the CE FLAIR involvement and RCVS severity defined by a composite outcome score calculated for each individual patient.
We graded the severity of RCVS by a composite neurological score that included PRES like edema appearance on MRI (0, 1), clinical seizures (0, 1), subarachnoid hemorrhage (0, 1), brain ischemia (0, 1) and thunderclap headache on initial presentation (0, 1). Multivariate logistic regression analysis was used to assure that the score components were not affected by demographic or clinical variables. The score was devised according to previously described markers of RCVS severity [6]. The grading of CE FLAIR included the composite of intensity of sulci enhancement by contrast (0, no signal; 1, for mild signal; 2, for substantial signal) with its distribution throughout the brain (1 point for each involved lobe—including cerebellar hemispheres; 0-10).
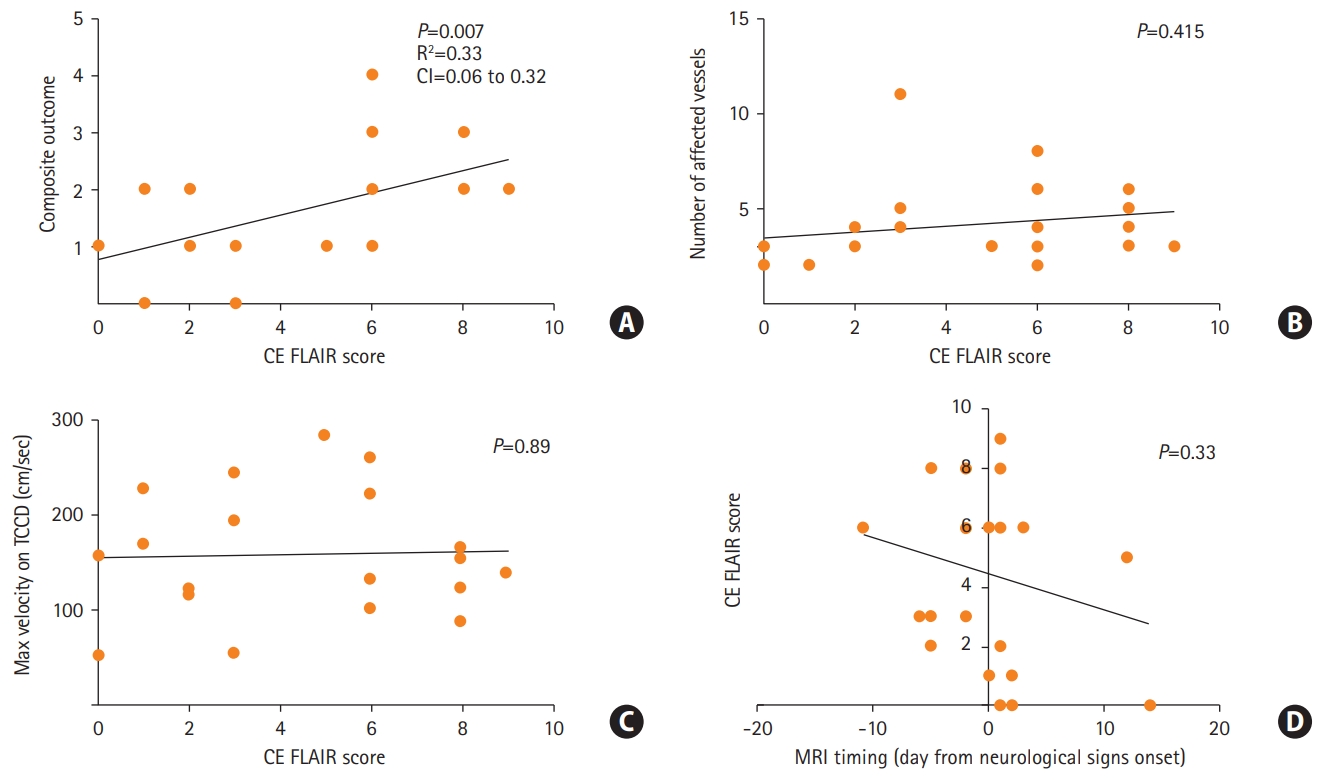
All the patients were female with a median age of 41, 17 (68%) with a non-significant prior medical history. Twenty-three patients (92%) were considered for an analysis (with available of MRI scans). Finally, 21 (85%) confirmed RCVS patients were included for the analysis. None of the patients exhibited a cellular inflammatory reaction in the CSF (Supplementary materials). All included patients underwent serial transcranial Doppler (TCD) imaging. Eighteen out of 21 confirmed RCVS patients exhibited increased CE FLAIR signal in cortical sulci (CE FLAIR score >0 in Supplementary Table 1, Supplementary materials). Sixteen of 21 patients suffered from neurological complications. In 18 out of 21 patients a putative causative trigger was isolated (Supplementary Table 1, Supplementary materials). Figure 2A shows that the composite score was significantly correlated with the enhancement severity on CE FLAIR signal (Pearson’s correlation analysis, Linear regression, R2=0.33, P=0.007) (Supplementary materials). However, neither number of affected vessels nor maximum velocity on TCD (surrogates of vasoconstriction) were correlated with the CE FLAIR score (Figure 2B and C), Pearson’s correlation analysis, P=0.415 and P=0.89, accordingly). In addition, symptom duration (indicated by timing of MRI acquisition) was not correlated with the CE FLAIR score (Pearson’s correlation analysis, P=0.33) (Figure 2D). In all cases of available follow-up MRI, CE FLAIR signal subsided to undetectable along with the resolution of vasospasm. A multivariate analysis exploring the association of demographic factors and neurological score components with the severity of CE FLAIR signal revealed only positive correlation with PRES like edema (effect estimate, 3.922; 95% confidence interval, 0.29 to 0.753; P=0.037) suggesting an overall more benign clinical course in our cohort in comparison to previous reports [5,6].
We suggest that contrasted enhancement on FLAIR imaging may reflect an early vasogenic process [7,8] of either delayed blood flow or local BBB disruption with a capillary leak as proposed recently in an earlier mentioned cohort [5] and in a later very compelling study, also of Asian population [6]. The earlier studies showed a correlation between leptomeningeal gadolinium enhancement on FLAIR imaging and neurological outcome, leading to diagnostic changes in unclear cases. While these studies were performed in an Asian population, our cohort was comprised of exclusively Jewish women, which provides a further validation of the findings.
In two most devastating cases, initial computed tomography angiography was normal or not performed, delaying correct diagnosis and appropriate treatment. A lack of correlation between CE FLAIR signal and degree of vasospasm supports a more complex role of putative vascular dysregulation in parenchymal involvement of RCVS.
We believe that this data represents an interesting radiological phenomenon in early RCVS that correlates to the clinical outcome of this rare but potentially devastating syndrome.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.01004.
Figure 1.
Grades of sulcal contrast enhancement (arrows) in four represenataive reversible cerebral vasoconstriction syndrome patients according to increasing enhancement severity. (A) Enhancement score of 1. (B) Enhancement score of 2. (C) Enhancement score of 2, different anatomic locus. (D) Enhancement score of 4.

Figure 2.
(A) Correlation of magnetic resonance imaging (MRI) severity score to composite neurological outcome score (Pearson’s correlation analysis). (B, C) Subanalysis of MRI fluid attenuation inversion recovery (FLAIR) correlation to the degree of vasospasm. (D) Correlation of MRI timing on MRI FLAIR enhancement (Pearson’s correlation analysis, negative values represent acquisition prior to development of vasospasm in days). CI, confidence interval; CE FLAIR, contrast enhanced FLAIR; TCCD, transcranial color Doppler.
References
1. Mehdi A, Hajj-Ali RA. Reversible cerebral vasoconstriction syndrome: a comprehensive update. Curr Pain Headache Rep 2014;18:443.



2. Singhal AB, Topcuoglu MA, Fok JW, Kursun O, Nogueira RG, Frosch MP, et al. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 2016;79:882-894.


3. Ducros A, Wolff V. The typical thunderclap headache of reversible cerebral vasoconstriction syndrome and its various triggers. Headache 2016;56:657-673.


4. Lee WJ, Yeon JY, Jo KI, Kim JS, Hong SC. Reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome presenting with deep intracerebral hemorrhage in young women. J Cerebrovasc Endovasc Neurosurg 2015;17:239-245.



5. Lee MJ, Cha J, Choi HA, Woo SY, Kim S, Wang SJ, et al. Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: implications for pathophysiology and diagnosis. Ann Neurol 2017;81:454-466.


6. Cho S, Ling YH, Lee MJ, Chen SP, Fuh JL, Lirng JF, et al. Temporal profile of blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome. Stroke 2020;51:1451-1457.










